Abstract
Background
Several guidelines support polypharmacy management in individual patients. More organisational-level focus is needed on the use of implementation frameworks.
Aim
To characterise the peer reviewed literature on implementation frameworks, focussing on barriers and facilitators to implementation at organisational level in the context of polypharmacy management.
Method
A scoping review protocol was devised, supporting retrieval of studies published in English, reporting from any sector of practice. Medline, International Pharmaceutical Abstracts, Cumulative Index of Nursing and Allied Health Literature and Business Source Complete were searched to January 2022 using Medical Subject Headings including: ‘polypharmacy’, ‘deprescriptions’, ‘strategic planning’ and ‘organizational innovation’. A narrative approach to data synthesis was applied. Searching, data extraction and synthesis were undertaken independently by two reviewers.
Results
After screening 797 records eight papers remained. Two were descriptive outlining details of specific initiatives, six used qualitative methods to explore determinants for implementation including barriers and enablers. Organisation level barriers included: poor organisational culture with a lack of sense of urgency and national plans, resource availability and communication issues including patient information and at transitions of care. Organisational facilitators included availability of government funding and regulatory environment promoting patient safety, a national emphasis on quality of care for older adults, co-ordinated national efforts and local evidence.
Conclusion
Limited literature focusses on the use of implementation frameworks at organisational levels. This review highlights the need for further work on implementation frameworks in this context to help achieve effective organisational change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
This scoping review highlights the importance of developing and using structured implementation frameworks and organizational change elements for polypharmacy management.
-
To contribute to safe, effective and economic patient care more research is needed on the use of organisational-level implementation frameworks for polypharmacy management.
-
A focus on local contexts and cultures should be considered to a greater extent to facilitate the development of implementation frameworks for organisation change.
-
More research is required on the impact of implementation frameworks and organisation change theory in the polypharmacy management context.
Introduction
The world’s population is ageing with associated increases in multimorbidity [1, 2] which can result in a rise in medication burden and hence polypharmacy [3]. The prevalence of polypharmacy around the world varies by country, ranging from 24.3% in Europe [4] to 40% in the USA [5] and is even higher in the Middle East [3, 6, 7]. Aitken and Gorokhovich reported that the global health expenditure could be reduced by 0.3% through managing inappropriate polypharmacy [8]. A systematic review and meta-analysis of observational studies examined adverse health outcomes and health care utilisation outcomes of polypharmacy in older adults and found consistent evidence for inappropriate prescribing and hospitalisation [9]. Previous European and North American studies suggest that polypharmacy is a complex global issue [10, 11].
There has been much focus on the development of clinical guidelines and recommendations for the management of inappropriate polypharmacy. A clinical guideline has been defined as ‘… recommendations on how healthcare and other professionals should care for people with specific conditions. The recommendations are based on the best available evidence.’ [12]. As such, guidelines are often focussed on the process of individualising patient care based on the best evidence and often do not consider the wider organisational context and implications for healthcare management.
A review of polypharmacy management guidance [13] identified that only five European Union (EU) countries had produced guidance documents focussing specifically on polypharmacy management in older people and few existed in other parts of the world. Only the Scottish Government Model of Care Polypharmacy Working Group Polypharmacy Guidance included consideration of the importance of inclusion of change management strategies that matches organisational contexts to ensure identification of all aspects relevant to effective implementation including barriers and facilitators.
Indeed, in 2017 the World Health Organization (WHO) addressed polypharmacy in the third Global Patient Safety Challenge “Medication without harm” [14] and advised countries to consider implementation of organisational change management strategies to overcome barriers to executing polypharmacy management programs [15]. The EU-funded project ‘Stimulating Innovative Management of Polypharmacy and Adherence in the Elderly’ (SIMPATHY) [16] highlighted the importance of theory-based guideline implementation frameworks for organisational change management [17].
Implementing guidelines effectively into practice is complex and challenging and Kitson et al. proposed a ‘conceptual framework’. They posited that there is often a predominant focus on the level and nature of evidence for guideline implementation and identified a requirement for an equal focus on other aspects such as the context and environment, the actual process of implementation and its facilitation [18]. More recently, Moullin et al. have published a systematic review of implementation frameworks for innovations in healthcare and have proposed a ‘Generic Implementation Framework’. This outlines core implementation concepts including the need for comprehensive information on: process of implementation (steps/stages), characteristics of the innovation, definition of the context, barriers and enablers and strategies for evaluation [19].
Having polypharmacy management guidelines that outline approaches for individual patient care can be of limited value [13]. Consideration of implementation frameworks incorporating polypharmacy management at organisational levels are therefore considered of prime importance as part of global concerted efforts to improve appropriate use of medicines [14]. Although there are numerous studies on the use of polypharmacy management strategies at the patient level, there is a paucity of information about the effective use of implementation frameworks for polypharmacy management at the healthcare organisational level.
It has been noted that social context can be a key facilitator of quality improvement and that there are structural levels within socio-institutional theory including: macro—the system level, meso—the organisation level and micro—the team or individual level [20].
Fulop and Roberts have defined the meso-structural level of the ‘organisation’ in healthcare as ‘health care entities at any level providing any kind of health care’ [20]. This is distinct from the health system level which encompasses aspects external to the organisational entities and which may include, for example, government level health and social care units such as ministries or governmental departments. Fulop and Robert also assert that the majority of factors influencing quality improvement success relate to the meso-structural organisation level and include: ‘leadership, cultures, climate, organisational experience of quality improvement, organisational size, financial and clinical performance, data and information systems, knowledge and training’ [20]. For these reasons this review focusses on the ‘organisational level’.
Aim
The aim of this review was to characterise the peer reviewed literature on implementation frameworks, with a focus on barriers and facilitators to implementation at organisational level in the context of polypharmacy management. It was designed to address the following questions (1) what are the characteristics of the literature including study aims, research designs, methods and study populations ? (2) what are the different characteristics of these frameworks, including the process of their development, structure and content, evaluation/monitoring and assessment of implementation outcomes? (3) what are the reported barriers and facilitators that influence the use of implementation frameworks for organisational change?
Method
A scoping review is often used when a research topic has not been widely explored and summarised through previous reviews [21]. They are useful for exploring topic areas where there is ambiguity around definitions and concepts which would make the use of other methods such as systematic review methodology challenging [22]. This scoping review followed the 6-stage methodological framework of Arksey and O’Malley to inform the conduct of the review [23]. Reporting was guided by the Preferred Reporting for Systematic and Meta-Analysis (PRISMA) extension for scoping reviews [24] and related guidance [25].
Eligibility criteria
Eligibility criteria and their rationale for inclusion and exclusion of studies in the review are presented in Table 1. This includes the detail of the population, concept and context of this review. The defined populations, concepts and contexts were broad to ensure consideration and inclusion of a wide range of literature.
Information sources
The following electronic databases were searched; Medline, International Pharmaceutical Abstract, Cumulative Index to Nursing and Allied Health Literature and Business Source Complete. No restriction was made on search dates with searches from database inception to January 2022.
Search strategy
Relevant keywords and Medical Subject Headings (MeSH) terms were used including: ‘polypharmacy’, ‘deprescriptions’, ‘strategic planning’ and ‘organizational innovation’. Combination of search terms, Boolean operators (such as OR, AND), and truncations (*) were used as appropriate to broaden the search and to retrieve all relevant papers (Table 1).
Literature source selection
To identify relevant papers, a wide range of literature was considered: full text peer reviewed papers reporting primary research, and descriptive and review articles. Grey literature, conference abstracts, protocols, book reviews, opinion articles, and editorials were excluded. Two researchers searched the databases independently using the agreed search strategy and cross-checked findings. All relevant studies were imported to RefWorks and duplicates removed. Initially two researchers independently screened the titles and abstracts of all retrieved papers against the pre-piloted inclusion criteria; papers were included if both reviewers agreed they should be. Full-text articles for all included papers were similarly reviewed. Any disagreements were adjudicated by a third member of the team.
Data charting process
The data fields to be extracted from the selected studies were agreed by the research team based on the scoping review aims and questions. A data extraction tool was developed and reviewed by the research team then modified based on a piloting and subsequently used to extract data from the selected full-text articles. Data extraction was conducted independently by two researchers and discussed with the entire review team.
The following information was recorded: authors, year of publication, title, aim/objectives, methods, characteristics of the implementation strategies and frameworks, setting, country, sector, specialty, professions of the participants, intervention findings, and barriers and facilitators to implementation.
Summary, synthesis, and reporting of results
The results of the scoping review are presented using a descriptive narrative approach to data synthesis.
Results
Selection of papers
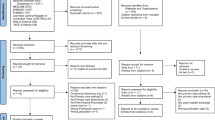
The PRISMA flow diagram in Fig. 1 provides information on the steps for selection of papers from the four databases. Of the 797 records identified after removal of duplicates, 723 were removed after title and abstract screening and a further 66 removed after full-text review. Eight remaining articles met the eligibility criteria.
Quantitative and textual summaries of the findings based on the extracted data are presented in Table 2.
Characteristics of the literature
Seven of the included studies were conducted between 2016 and 2020 [17, 26,27,28,29,30, 32]. Studies included addressed mainly the management of polypharmacy. They focused on: the availability of policies and guidelines on polypharmacy management in older people [26, 32]; the characteristics of healthcare professionals’ barriers and facilitators to polypharmacy management [26,27,28]; and the development of frameworks of interventions to facilitate the implementation and sustainability of polypharmacy management programs [17, 26, 29].
Two papers provided detailed descriptions of the characteristics of programs that addressed the care of frail older people but did not carry out data collection [30, 31]. In terms of research design, the other 6 papers [17, 26,27,28,29, 32] mainly used qualitative methods including: interviews [17, 27, 28], focus groups [17, 26, 27] and Delphi consensus [29] and workshops [32].
In relation to study populations five of the studies that were conducted in European countries [17, 26, 27, 29, 30]. One was conducted in the United States [31] and two in Australia [28, 32]. Three studies [17, 29, 32] were conducted at national levels with two of these related to the EU funded SIMPATHY project [17, 29] and the other conducted in conjunction with Australian Deprescribing Network [32]. Other papers focused on the healthcare organisational level of: primary care [28, 31], hospital inpatient only [26] and inpatient and outpatient care [27]. The two descriptive studies did not specify a focus but seemed to take a cross-sectoral approach [30, 31].
The two studies from the SIMPATHY project involved stakeholders (clinicians and managers) working in policy development and implementation [17, 29]. One paper involved professional groups, academia, aged care organisations and regulatory agencies [32]. Other involved geriatricians [26], pharmacists [26,27,28], general practitioners [27, 28], medical specialists [27], nurses [27, 28], and medical assistants [27].
Synthesis of characteristics of implementation frameworks
Articles included different implementation strategies and frameworks for polypharmacy management which included details of development, structure and content, evaluation, monitoring, and assessment of implementation outcomes. Strategies suggested for effective change management included the Plan-Do-Study-Act (PDSA) [30], Kotter’s eight-step change model [17, 29], and Normalization Process Theory (NPT) to evaluate implementation processes [17]. Scott et al. used the Theoretical Domains Framework (TDF) to identify suitable behaviour change techniques to inform hospital de-prescribing frameworks for polypharmacy management [26]. The American Geriatrics Society (AGS) Guiding Principles for the Care of Older Adults with Multimorbidity was used by Australian researchers as a framework for analysis and informed the process of designing domains related to multimorbidity management [28] and Kouladjian O’Donnell et al. synthesised findings of a workshop into recommendations for an Australian national strategic action plan (rNSAP) to reduce inappropriate polypharmacy [32]. One study used a translational research framework, Promoting Action on Research Implementation in Health Services (PARIHS), as a tool to translate a medication management model into practice settings [31]. Straßner et al. used the ‘Tailoring Interventions for Chronic Diseases (TICD) checklist’, a comprehensive framework of determinants of practice to improve the care of patients with multimorbidity and polypharmacy. This approach was developed and evaluated in a cluster-randomised controlled trial, but little information was presented regarding the framework development and implementation [27].
Synthesis of barriers and facilitators to implementation
The literature included in this review highlights the many barriers that can affect implementation of polypharmacy guidelines. Many of these were at the organisational-level and so very relevant to this review. However, others were at health system level, healthcare professional and individual patient levels and in view of the integrated nature of the review many are considered here.
Health system-level barriers included a lack of data which would facilitate the creation of a sense of urgency, the absence of national plans for implementation, monitoring, and evaluation, geographically and culturally diverse settings for implementation, and operational differences across implementing sites [17, 28, 30].
Barriers at the organisational level included: poor medication safety culture and resource availability [17, 26], system limitations, lack of availability of information for patients and transfer at transitions of care [26]. Poor communication systems were also highlighted as impeding implementation [17, 28] as did a lack of time for practice e.g. time for conducting structured medication reviews [27]. Organisational and healthcare system level facilitators included availability of government funding streams and strict regulatory environments, a national emphasis on quality of care for older adults and co-ordinated national efforts [17, 29, 30, 32].
Healthcare professional focussed barriers, many of which arose from organisational issues included: poor care coordination, lack of time, unclear allocation of tasks and responsibilities, lack of required competencies for pharmacists to make decisions, educational and training differences among staff, and a lack of shared decision making among healthcare professionals [27, 28, 30]. Barriers at the patient level were around social influences such as patient perceptions, expectations, and preferences in relation to medication [26]. Patient level facilitators included levels of patient access to healthcare resources, patient negative experiences of medications and perceptions around improved outcomes for patients [26].
Discussion
Statement of key findings
This review summarises literature on frameworks used and barriers and facilitators for their implementation for change, in relation to polypharmacy management, at organisational levels. The majority of articles were published between 2016 and 2020 with an array of geographic locations. Two were descriptive papers outlining details of specific initiatives, five papers used a range of qualitative methods to explore the rationale for the development of polypharmacy management initiatives and determinants for the implementation including barriers and enablers. Organisational level barriers included: poor organisational culture with a lack of sense of urgency and national plans, resource availability and communication issues including patient information and transition of care. Organisational facilitators included availability of government funding and regulatory environment promoting patient safety, a national emphasis on quality of care for older adults, co-ordinated national efforts and local evidence. This review shows that while polypharmacy management guidelines have been developed in some countries, there has been a limited focus on the development and implementation of frameworks, especially at the organisational level. There was limited literature for the use of implementation frameworks for polypharmacy management at the organisational level.
Strengths and limitations
This is the first scoping review that synthesises literature related to organisational change in polypharmacy management. This review focussed on peer reviewed published literature and as such excluded grey literature, conference abstracts, protocols, book reviews, opinion articles and editorial reviews. In view of this, some papers may have been omitted but the authors felt this focus was important and appropriate given the aims of the review. The search strategy included two overarching terms relevant to the review aim along with a range of over 29 sub-terms. It is possible that other terms could have been included but the use of MESH® terms and explosion of subject headings within the search databases ensured comprehensive inclusion of paper for screening. This review included only articles published in English as there was no resource available for translation services. It is also acknowledged that there are many factors that can influence quality improvement in relation to medication burden. The review does not attempt to cover the many facets of quality medicines use of which polypharmacy is acknowledged to be only one.
Interpretation
Common organisational contextual factors influencing the processes of quality improvement approaches were identified in papers in Kaplan et al’s systematic review, these included: organisational characteristics (e.g., size, ownership), leadership from top management, competition, organisational culture and data infrastructure/information systems [33]. These were considered to be broadly similar to constructs included in established theories of implementation and organisational change [34, 35].
Given this, an approach within implementation science to address such organisational factors is to consider how ‘frameworks’ can be used to help support innovation. Bauer et al. define ‘frameworks’ as ‘… a set of constructs that organise concepts and data … provide a prescriptive series of steps summarising how implementation should ideally be planned and carried out.’ [36]. Such frameworks offer a structured, robust method for operationalising and evaluating innovation.
Co-ordinated national efforts focussing on organisations and use of frameworks for implementation are highlighted from the studies by McIntosh et al. [17], McNamara et al. [28], Alkema and Frey [31] and Kouladjian et al. [32]. These show that factors that enable the implementation of polypharmacy strategies include: governmental support, availability of policies and legislations, funding, strict regulations and availability of good quality data. This is also supported by McIntosh et al. [37], Gennimata et al. [38], and Kempen [39] who highlighted a national focus on quality of care for older adults as well as the existence of health policies focusing on improved care for patients with complex chronic diseases. This is important since it has been shown that for implementation of evidence-based practice it is important to have a focus on the level and nature of evidence for guideline implementation along with an equal focus on other aspects such as the context and environment [18].
The papers by Conroy et al. [30] and Alkema and Frey [31] were descriptive in nature. Each outlined details of quite different projects with relevance to implementation of polypharmacy initiatives and offer vital information concerning this. Moullin et al. in their systematic review of implementation frameworks highlight the need to give consideration to: process of implementation (steps/stages), characteristics of the innovation, definition of the context, barriers and enablers and strategies for evaluation [19].
Despite the variety of methods used within included papers there was a clear message that polypharmacy continues to be considered an important issue that can result in improvements in the care of older people [30, 32] and have positive impacts through utilisation of change management frameworks at the organisational level [17, 31]. This is supported by Kempen et al. who have stated that ‘.organisational change strategy is a key factor involved in the implementation and sustainability of polypharmacy management programs’ [39].
The importance of consideration of context and environment within conceptual frameworks for implementation of initiatives has been highlighted [18]. Some papers highlighted the fact that challenges during the implementation process arose from barriers either at organisational level, including a lack of clear responsibilities for who conducts polypharmacy reviews, or within hospitals where initiatives were not considered as a priority [26, 30]. Similar barriers were demonstrated by Kempen et al. [39], who reported that a lack of attention when integrating new practices into daily workflows is also a barrier to implementing polypharmacy management programs. Gennimata [38] reported extreme financial pressure, a lack of organisational culture supporting multidisciplinary teams, and a lack of shared decision making and leadership from central health authorities as a barrier to implementation of polypharmacy management. The work by McIntosh et al. [17] and Stewart et al. [29] noted that without a fully coordinated strategy taking into consideration change management the desired outcomes are often not fully achieved and sustained across a population irrespective of the clarity and robustness of specific innovation.
Structured organisational change strategies are generally considered to enhance the data collection and implementation processes. These are entirely compatible with the principles of implementation frameworks where there is an expectation to clearly define aspects such as process of implementation, characteristics of the innovation, context and barriers and enablers [19]. Such organisational change strategies were evident in the included papers with the use of Kotter’s eight-step process used in the SIMPATHY project [29], Plan-Do-Study-Act quality improvement cycle approach by Conroy et al. [30], and the use by Alkema and Frey, of a translational research framework promoting action on research implementation in health services [31]. However, none of the studies specifically mentioned development and implementation of polypharmacy management frameworks.
The literature identified for inclusion in this review helps provide insights to the approaches taken to use structured approaches to address the introduction of polypharmacy management at organisational levels. Fulop and Robert proposed that there should be co-design and dissemination of tools, such as implementation frameworks [19] that enable organisational factors to be taken into account before beginning improvement interventions. In turn this would support relevant, contextualised intervention development which is systematically embedded within these implementation frameworks [20].
Further research
Further research should focus on the development and testing of implementation frameworks at the meso-structural organisational level. Initial work could focus on definitions relating to concepts and contexts to facilitate cross-country and sector comparisons. This could be followed by consensus-based approaches for the development of context specific, theory-based implementation frameworks. Specific interventions to operationalise the frameworks could then be developed, implemented and evaluated with cognisance of the Medical Research Council guidance on developing and evaluating complex interventions [40].
Conclusion
Although initiatives and guidelines for polypharmacy management are available, this review demonstrates that there is a lack of research focussed on implementation frameworks and how they can be used for change at organisational levels. Implementation of polypharmacy management programs are unlikely to fully achieve the desired outcomes unless implementation frameworks for organisational change are more fully considered.
References
Hughes LD, McMurdo ME, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing. 2013;42(1):62–9.
Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Rev. 2010;32(2):451–74.
Al-Dahshan A, Al-Kubiasi N, Al-Zaidan M, et al. Prevalence of polypharmacy and the association with non-communicable diseases in Qatari elderly patients attending primary healthcare centers: a cross-sectional study. PLoS ONE. 2020;15(6):e0234386. https://doi.org/10.1371/journal.pone.0234386.
Onder G, Pedone C, Landi F, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriat Soc. 2002;50(12):1962–8.
Dwyer LL, Han B, Woodwell DA, et al. Polypharmacy in nursing home residents in the United States: results of the 2004 national nursing Home Survey. Am J Geriatr Pharmacother. 2010;8(1):63–72.
Al-Hashar A, Al Sinawi H, Al Mahrizi A, et al. Prevalence and covariates of polypharmacy in elderly patients on discharge from a tertiary care hospital in Oman. Oman Med J. 2016;31(6):421–5.
Alsuwaidan A, Almedlej N, Alsabti S, et al. A comprehensive overview of polypharmacy in elderly patients in Saudi Arabia. Geriatrics. 2019;4(2):36.
Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN. 2012. https://ssrn.com/abstract=2222541. Accessed 11 Nov 2022.
Davies LE, Spiers G, Kingston A, et al. Adverse outcomes of polypharmacy in older people: systematic review of reviews. JAMDA. 2020;21(2):181–7.
Cadogan CA, Ryan C, Francis JJ, et al. Development of an intervention to improve appropriate polypharmacy in older people in primary care using a theory-based method. BMC Health Serv Res. 2016;16(1):661.
Anderson LJ, Schnipper JL, Nuckols TK, et al. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. AJHP. 2019;76(21):1777–87.
NICE The guidelines manual Process and methods [PMG6]. 2012. https://www.nice.org.uk/process/pmg6/resources/how-nice-clinical-guidelines-are-developed-an-overview-for-stakeholders-the-public-and-the-nhs-2549708893/chapter/nice-clinical-guidelines Accessed 11 Nov 2022.
Stewart D, Mair A, Wilson M, et al. SIMPATHY consortium. Guidance to manage inappropriate polypharmacy in older people: systematic review and future developments. Expert Opin Drug Saf. 2017;16(2):203–13.
Donaldson LJ, Kelley ET, Dhingra-Kumar N, et al. Medication without harm: WHO’s third global patient safety challenge. Lancet. 2017;389(10080):1680–1.
World Health Organization. Medication safety in polypharmacy: technical report. World Health Organization; 2019. https://www.who.int/publications/i/item/WHO-UHC-SDS-2019.11 Accessed 11 Nov 2022.
Mair A, Fernandez-Llimos F, Alonso A et al. Simpathy Consortium. Polypharmacy management by 2030: a patient safety challenge. https://ec.europa.eu/chafea/health/newsroom/news/documents/polypharmacy-handbook-second-edition_en.pdf Accessed 11 Nov 2022.
McIntosh J, Alonso A, MacLure K, et al. A case study of polypharmacy management in nine european countries: implications for change management and implementation. PLoS ONE. 2018;13(4):e0195232. https://doi.org/10.1371/journal.pone.0195232.
Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care. 1998;7(3):149–58.
Moullin JC, Sabater-Hernández D, Fernandez-Llimos F, et al. A systematic review of implementation frameworks of innovations in healthcare and resulting generic implementation framework. Health Res Policy Sys. 2015;13:16.
Fulop N, Robert G. Context for successful quality improvement: Evidence review. The Health Foundation. 2015. https://www.health.org.uk/publications/context-for-successful-quality-improvement . Accessed 11 Nov 2022.
Peters MD, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6.
Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Implement. 2021;19(1):3–10.
Scott S, Twigg MJ, Clark A, et al. Development of a hospital deprescribing implementation framework: a focus group study with geriatricians and pharmacists. Age Ageing. 2020;49(1):102–10.
Straßner C, Steinhäuser J, Freund T, et al. German healthcare professionals’ perspective on implementing recommendations about polypharmacy in general practice: a qualitative study. Fam Pract. 2018;35(4):503–10.
Mc Namara KP, Breken BD, Alzubaidi HT, et al. Health professional perspectives on the management of multimorbidity and polypharmacy for older patients in Australia. Age Ageing. 2017;46(2):291–9.
Stewart D, Gibson-Smith K, MacLure K, et al. A modified Delphi study to determine the level of consensus across the European Union on the structures, processes and desired outcomes of the management of polypharmacy in older people. PLoS ONE. 2017;12(11):e0188348. https://doi.org/10.1371/journal.pone.0188348. Accessed 11 Nov 2022.
Conroy S, Thompson D, Griffiths S, et al. Improving acute care for older people at scale-the acute frailty network. Acute Med. 2016;15(4):185–92.
Alkema GE, Frey D. Implications of translating research into practice: a medication management intervention. Home Health Care Serv Q. 2006;25(1–2):33–54.
Kouladjian O’Donnell L, Reeve E, Cumming A, et al. Development and dissemination of the national strategic action plan for reducing inappropriate polypharmacy in older Australians. Int Med J. 2021;51(1):111–5.
Kaplan HC, Brady PW, Dritz MC, et al. The influence of context on quality improvement success in health care: a systematic review of the literature. Milbank Q. 2010;88(4):500–59.
Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of Health Services Research Findings into Practice: a Consolidated Framework for advancing implementation science. Implement Sci. 2009;4:50.
Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629.
Bauer MS, Damschroder L, Hagedorn H, et al. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32. https://doi.org/10.1186/s40359-015-0089-9. Accessed 11 Nov 2022.
McIntosh JA, Alonso A, Codina C. Polypharmacy and adherence: key components of integrated care, the case of Catalonia. Int J Integr Care. 2016;16(6):A32. https://doi.org/10.5334/ijic.2975. Accessed 11 Nov 2022.
Gennimata D, Kampolis CF, Vontetsianos T, et al. Medication review and polypharmacy management in the hospital setting. The cases of Greece and Catalonia. Int J Integr Care. 2017;17(5):550. https://doi.org/10.5334/ijic.3870. Accessed 11 Nov 2022.
Kempen TG, Gillespie U, Färdborg M, et al. A case study of the implementation and sustainability of medication reviews in older patients by clinical pharmacists. Res Social Adm Pharm. 2019;15(11):1309–16.
Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. https://doi.org/10.1136/bmj.n2061.
Acknowledgements
None.
Funding
This scoping review did not receive any funding from any sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Derek Stewart is the Editor-in-Chief of the International Journal of Clinical Pharmacy. He had no role in handling the manuscript, specifically the processes of editorial review, peer review and decision making.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Bulushi, S., McIntosh, T., Grant, A. et al. Implementation frameworks for polypharmacy management within healthcare organisations: a scoping review. Int J Clin Pharm 45, 342–354 (2023). https://doi.org/10.1007/s11096-023-01534-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01534-8