Abstract
Background
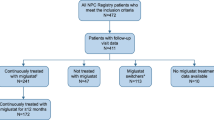
Niemann–Pick disease type C (NP-C) is a progressive neurodegenerative disorder with early infantile (< 2 years), late infantile (2–6 years), juvenile (7–15 years) and adolescent (> 15 years) onset. The mainstay of therapy for NP-C patients with neurological symptoms is miglustat, a drug that may modify the course of the disease.
Aim
Our aim was to evaluate the cost-effectiveness of miglustat in comparison to symptomatic therapy in patients with NP-C in the socio-economic settings of the Republic of Serbia, an upper-middle-income European economy.
Method
The perspective of the Serbian Republic Health Insurance Fund was chosen for this study, and the time horizon was eighty years. The main outcomes of the study were quality-adjusted life years gained with miglustat and comparator, and direct costs of treatment. The study was conducted through the generation and simulation of the Discrete-Event Simulation model. The model results were obtained after Monte Carlo microsimulation of a sample with 1000 virtual patients.
Results
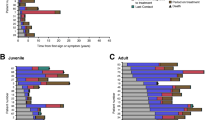
Treatment with miglustat was not cost-effective when compared with symptomatic therapy and was associated with negative values of net monetary benefit regardless of the onset of neurological manifestations (− 110,447,627.00 ± 701,614.00 RSD, − 343,871,695.00 ± 2,577,441.00 RSD, − 1,397,908,502.00 ± 23,084,235.00 RSD and − 2,953,680,879.00 ± 33,297,412.00 RSD) for early infantile, late infantile, juvenile and adolescent cohorts, respectively).
Conclusion
When traditional pharmacoeconomic evaluation is employed, miglustat is not a cost-effective option in comparison to symptomatic therapy for the treatment of NP-C. However, given the proven efficacy of miglustat, there is a need to find ways to make this drug available to all patients with NP-C.




Similar content being viewed by others
References
Sitarska D, Tylki-Szymańska A, Ługowska A. Treatment trials in Niemann–Pick type C disease. Metab Brain Dis. 2021;36(8):2215–21.
Geberhiwot T, Moro A, Dardis A, et al. Consensus clinical management guidelines for Niemann–Pick disease type C. Orphanet J Rare Dis. 2018;13(1):50.
Bajwa H, Azhar W. Niemann–Pick Disease. [Updated 2021 Jul 18]. In: StatPearls [Internet]. Treasure island (FL): StatPearls Publishing; 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK556129/. Accessed 28 Feb 2022.
Kwon HJ, Abi-Mosleh L, Wang ML, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137(7):1213–24.
Infante RE, Wang ML, Radhakrishnan A, et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105(40):15287–92.
Maresca G, Formica C, Nocito V, et al. Neuropsychological assessment in Niemann–Pick disease type C: a systematic review. Neurol Sci. 2021;42(8):3167–75.
Vanier MT. Niemann–Pick disease type C. Orphanet J Rare Dis. 2010;5:16.
Seker Yilmaz B, Baruteau J, Rahim AA, et al. Clinical and molecular features of early infantile Niemann Pick type C disease. Int J Mol Sci. 2020;21(14):5059.
Imrie J, Galani C, Gairy K, et al. Cost of illness associated with Niemann–Pick disease type C in the UK. J Med Econ. 2009;12(3):219–29.
Pineda M, Walterfang M, Patterson MC. Miglustat in Niemann–Pick disease type C patients: a review. Orphanet J Rare Dis. 2018;13(1):140.
de Souza MV, Krug BC, Picon PD, et al. High cost drugs for rare diseases in Brazil: the case of lysosomal storage disorders. Cien Saude Colet. 2010;15(Suppl 3):3443–54.
Kanters TA, van der Ploeg AT, Kruijshaar ME, et al. Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in adult patients with pompe disease. Orphanet J Rare Dis. 2017;12(1):179.
Darbà J, Marsà A. Current status and use of resources of lysosomal storage diseases: analysis of a spanish claims database. Endocr Metab Immune Disord Drug Targets. 2020;20(2):263–70.
Davari M, Nabizadeh A, Kadivar M, et al. Healthcare resource utilization and the cost of care for mucopolysaccharidosis I patients in Iran. Value Health Reg Issues. 2019;18:165–9.
Walterfang M, Chien YH, Imrie J, et al. Dysphagia as a risk factor for mortality in Niemann–Pick disease type C: systematic literature review and evidence from studies with miglustat. Orphanet J Rare Dis. 2012;7:76.
WDI - Classifying countries by income. Available at: https://datatopics.worldbank.org/world-development-indicators/stories/the-classification-of-countries-by-income.html. Accessed 05 Feb 2022.
Parliament of republic of serbia. health insurance law. Off Gaz RS. 2019. Available at: https://www.paragraf.rs/propisi/zakon_o_zdravstvenom_osiguranju.html. Accessed 05 Feb 2022.
Imrie J, Heptinstall L, Knight S, et al. Observational cohort study of the natural history of Niemann–Pick disease type C in the UK: a 5-year update from the UK clinical database. BMC Neurol. 2015;15:257.
Republic Health Insurance Fund of Serbia. Rulebook on the prices of health services at the secondary and tertiary levels of health care. Off Gaz RS. 55/2019, 53/2021.
Republic Health Insurance Fund of Serbia. Rulebook on the List of Medicines Prescribed and Issued at the Expense of Compulsory Health Insurance Funds. Off Gaz RS. 43/19, 55/19, 56/19-correction, 73/19, 87/19, 18/20, 43 / 20, 108/20, 49/21, 51 / 21-correction and 60/21.
The Government of the Republic of Serbia. Decision on the highest prices of prescription medicines for human use. Off Gaz RS. 48/2021.
National Bank of Serbia. Interest rates. [Internet]. 2021. Available at: https://www.nbs.rs/sr/ciljevi-i-funkcije/monetarna-politika/kamatne-stope/. Accessed 05 Feb 2022.
Karnon J, Ali Afzali Haji H. When to use discrete event simulation (DES) for the economic evaluation of health technologies a review and critique of the costs and benefits of DES. Pharmacoeconomics. 2014;32(6):547–58.
Jankovic SM. Discrete-event simulation model for comparation of cost-effectiveness of miglustat versus symptomatic therapy in patients with Niemann–Pick disease type C. 2022. Available at: https://drive.google.com/file/d/1GshYch8Pz8ZvbKL6mJDrVHUGqqSBEWVV/view?usp=sharing. Accessed 09 Aug 2022.
Bianconi SE, Hammond DI, Farhat NY, et al. Evaluation of age of death in Niemann–Pick disease, type C: utility of disease support group websites to understand natural history. Mol Genet Metab. 2019;126(4):466–9.
Sevin M, Lesca G, Baumann N, et al. The adult form of Niemann–Pick disease type C. Brain. 2017;130(Pt 1):120–33.
Patterson MC, Vecchio D, Prady H, et al. Miglustat for treatment of Niemann–Pick c disease: a randomised controlled study. Lancet Neurol. 2007;6(9):765–72.
Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life–a population-based study. Aliment Pharmacol Ther. 2008;27(10):971–9.
Bremova T, Malinová V, Amraoui Y, et al. Acetyl-dl-leucine in Niemann–Pick type C: a case series. Neurology. 2015;85(16):1368–75.
Vergeer M, de Ranitz-Greven WL, Neary MP, et al. Epilepsy, impaired functioning, and quality of life in patients with tuberous sclerosis complex. Epilepsia Open. 2019;4(4):581–92.
Chittrakul J, Siviroj P, Sungkarat S, et al. Multi-system physical exercise intervention for fall prevention and quality of life in pre-frail older adults: a randomized controlled trial. Int J Environ Res Public Health. 2020;17(9):E3102.
Roberts J, Lenton P, Keetharuth AD, et al. Quality of life impact of mental health conditions in England: results from the adult psychiatric morbidity surveys. Health Qual Life Outcomes. 2014;12:6.
Giebel CM, Sutcliffe C, Stolt M, et al. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: a European study. Int Psychogeriatr. 2014;26(8):1283–93.
Majsiak E, Choina M, Golicki D, et al. The impact of symptoms on quality of life before and after diagnosis of coeliac disease: the results from a Polish population survey and comparison with the results from the United Kingdom. BMC Gastroenterol. 2021;21(1):99.
Varni JW, Nutakki K, Swigonski NL. Speech difficulties and patient health communication mediating effects on worry and health-related quality of life in children, adolescents, and young adults with Neurofibromatosis type 1. Am J Med Genet A. 2019;179(8):1476–82.
Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–12.
Cupurdija V, Lazic Z, Petrovic M, et al. Community-acquired pneumonia: economics of inpatient medical care vis-à-vis clinical severity. J Bras Pneumol Publicacao Of Soc Bras Pneumol E Tisilogia. 2015;41(1):48–57.
Hirsch AW, Monuteaux MC, Fruchtman G, et al. Characteristics of children hospitalized with aspiration pneumonia. Hosp Pediatr. 2016;6(11):659–66.
Mikulić I, Likić R, Janković SM. Cost-effectiveness of zonisamide versus levetiracetam in newly diagnosed focal onset epilepsy in Serbia. Value Health Reg Issues. 2022;27:49–57.
Davis JC, Dian L, Khan KM, et al. Cognitive status is a determinant of health resource utilization among individuals with a history of falls: a 12-month prospective cohort study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2016;27(3):943–51.
Marić NP, AndrićPetrović S, Jerotić S, et al. Maintenance phase treatment of psychotic disorders in outpatients from Serbia–focus on long-term benzodiazepine use. Int J Psychiatry Clin Pract. 2020;24(3):315–21.
Wimo A, Jönsson L, Gustavsson A, et al. The economic impact of dementia in Europe in 2008-cost estimates from the Eurocode project. Int J Geriatr Psychiatry. 2011;26(8):825–32.
Wells NE, Hahn BA, Whorwell PJ. Clinical economics review: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11(6):1019–30.
Flik CE, Laan W, Smout AJPM, et al. Comparison of medical costs generated by IBS patients in primary and secondary care in the Netherlands. BMC Gastroenterol. 2015;15:168.
Kaipa R, Peterson AM. A systematic review of treatment intensity in speech disorders. Int J Speech Lang Pathol. 2016;18(6):507–20.
Pineda M, Juríčková K, Karimzadeh P, et al. Disease characteristics, prognosis and miglustat treatment effects on disease progression in patients with Niemann–Pick disease type C: an international, multicenter, retrospective chart review. Orphanet J Rare Dis. 2019;14(1):32.
Patterson MC, Mengel E, Vanier MT, et al. Treatment outcomes following continuous miglustat therapy in patients with Niemann–Pick disease type C: a final report of the NPC registry. Orphanet J Rare Dis. 2020;15(1):104.
Zavesca prices, coupons & patient assistance programs. Drugs.com. Available at: https://www.drugs.com/price-guide/zavesca. Accessed 05 Feb 2022.
Statistical office of the republic of Serbia 2022 . Available at: https://data.stat.gov.rs/Home/Result/09020101?languageCode=sr-Cyrl. Accessed 12 Feb 2022.
Food and drug administration. ZAVESCA® (miglustat) capsules, for oral use. 2003. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021348s010lbl.pdf. Accessed 12 Feb 2022
Santos-Lozano A, Villamandos García D, Sanchis-Gomar F, et al. Niemann–Pick disease treatment: a systematic review of clinical trials. Ann Transl Med. 2015;3(22):360.
Solomon BI, Smith AC, Sinaii N, et al. Association of miglustat with swallowing outcomes in Niemann–Pick disease, type C1. JAMA Neurol. 2020;77(12):1564–8.
Nadjar Y, Hütter-Moncada AL, Latour P, et al. Adult Niemann–Pick disease type C in France: clinical phenotypes and long-term miglustat treatment effect. Orphanet J Rare Dis. 2018;13(1):175.
Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann–Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 2010;99(4):351–7.
Caso-González A, Núñez-Rodríguez J, Nebot-Villacampa MJ, et al. Experiencia clínica con medicamentos huérfanos para enfermedades raras metabólicas [clinical experience with orphan drugs for rare metabolic diseases]. An Pediatr (Engl Ed). 2020;S1695–4033(20):30437–9.
Holmerová I, Hort J, Rusina R, et al. Costs of dementia in the Czech Republic. Eur J Health Econ. 2017;18(8):979–86.
Statista. Health, Pharma & Medtech. Health Professionals & Hospitals. Average cost of inpatient day at U.S. hospitals in 2019, by hospital type. Available at: https://www.statista.com/statistics/630443/inpatient-day-hospital-costs-in-us-by-nonprofit-or-profit/. Accessed 28 Feb 2022.
Kovács G, Almási T, Millier A, et al. Direct healthcare cost of schizophrenia–European overview. Eur Psychiatry. 2018;48:79–92.
Burns ER, Stevens JA, Lee R. The direct costs of fatal and non-fatal falls among older adults–United States. J Safety Res. 2016;58:99–103.
Cannizzo S, Lorenzoni V, Palla I, et al. Rare diseases under different levels of economic analysis: current activities, challenges and perspectives. RMD Open. 2018;4(Suppl 1):e000794.
Javaid MK, Forestier-Zhang L, Watts L, et al. The RUDY study platform—a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis. 2016;11(1):150.
Murphy SM, Puwanant A, Griggs RC. Consortium for clinical investigations of neurological channelopathies (CINCH) and Inherited neuropathies consortium (INC) Consortia of the rare disease clinical research network. Unintended effects of orphan product designation for rare neurological diseases. Ann Neurol. 2012;72(4):481–90.
Sequeira AR, Mentzakis E, Archangelidi O, et al. The economic and health impact of rare diseases: a meta-analysis. Health Policy Technol. 2021;10(1):32–44.
Iskrov G, Stefanov R. Criteria for drug reimbursement decision-making: an emerging public health challenge in Bulgaria. Balkan Med J. 2016;33(1):27–35.
Danzon PM. At what price? Nature. 2007;449(7159):176–9.
Acknowledgements
The authors would like to thank dr Žan Friščić for his assistance with English grammar and syntax editing.
Funding
This study was partially financed by grant No 175007, given by the Serbian Ministry of Education, Science and Technological Development.
Conflicts of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gutić, M., Milosavljević, M.N. & Janković, S.M. Cost-effectiveness of miglustat versus symptomatic therapy of Niemann–Pick disease type C. Int J Clin Pharm 44, 1442–1453 (2022). https://doi.org/10.1007/s11096-022-01491-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01491-8