Abstract
Background
Transplant recipients undergo significant changes in their medication regimen during follow-up and are at an increased risk for medication-related problems (MRPs).
Aim
This study aimed to compare the prevalence and types of MRPs and interventions in liver transplant recipients with and without an outpatient medication consultation by a clinical pharmacist as well as the satisfaction with information about medicines and medication adherence.
Method
We performed a single-center, observational cohort study. A retro- and prospective cohort were used and subdivided in a group that did and did not receive a medication consultation. The prevalence and types of MRPs and interventions were identified and categorized. The satisfaction parameters were evaluated using validated questionnaires.
Results
Included were 291 patients. In total, 368 MRPs were identified in 197 patients in the non-medication consultation cohort (median 1; range 1–3 per patient) and 248 MRPs in 94 patients in the medication consultation cohort (median 2; range 1–4 per patient). In the medication consultation cohort, significantly fewer MRPs as unnecessary drugs (17.3% versus 58.7%, p < 0.001), suboptimal therapy (2.4% versus 9.5%, p < 0.001), untreated indication (2.8% versus 6.8%, p = 0.040) and underdosed drugs (0.4% versus 6.3%, p < 0.001) were identified. In the non-medication consultation cohort significantly more patients used unnecessary drugs (72.1% versus 39.4%, p < 0.001) compared to the medication consultation cohort. Patients in both cohorts are satisfied with the information about medicines and reported a high medication adherence.
Conclusion
Patients in the medication consultation cohort had significantly fewer MRPs and used significantly less unnecessary drugs. Including a clinical pharmacist to the post-transplant care has an added value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Transplant recipients undergo significant changes in their medication regimen during follow-up resulting in an increased risk for medication-related problems.
-
Including an outpatient medication consultation by a clinical pharmacist in the post-transplant care results in significantly fewer medication-related problems as unnecessary drugs, suboptimal therapy, untreated indications and underdosed drugs.
-
Since clinical pharmacists bring different perspectives to the post-transplant care, including one in the multidisciplinary transplant team has an added value for improving the pharmaceutical care, keeping the post-transplant care sustainable and optimizing medication safety in these patients.
Introduction
Liver transplantation (LT) has become a lifesaving treatment option for patients with end-stage liver disease, hepatocellular carcinoma and acute liver failure [1]. Over the past decades significant developments have been made in the field of LT, which steadily led to improved outcomes and long-term survival [1, 2]. However, long-term care after LT remains complex. LT recipients undergo significant changes in their medication regimen during follow-up with an increased risk for medication-related problems (MRPs).
LT recipients need to adhere to difficult and complex therapeutic regimens[2,3,4,5]. In addition, LT recipients will usually receive more medication over the years due to the development of new-onset diabetes mellitus, hypertension, and hyperlipidemia [6, 7]. The addition of more medication could cause MRPs and possibly result in preventable drug-related hospital admissions [8, 9]. A study by Repp et al. reported that 40% of the hospital admissions following cardiac transplantation were drug-related of which 58% was preventable [10].
Hepatologists focus mainly on the liver-related problems and transplant-specific complications. Clinical pharmacists involved in the transplant care provide a broad range of different services in order to prevent MRPs such as therapeutic drug monitoring, educational activities, management of adverse drug reactions (ADRs), dosing issues and therapy optimizations [11].
Recently, we showed the added value of an outpatient monitoring program for LT recipients by a clinical pharmacist through signaling relevant discrepancies and MRPs [12]. However, no studies have been done to compare the prevalence of MRPs and interventions in patients with and without an outpatient medication consultation (MC) by a clinical pharmacist.
Aim
This study aimed to compare the prevalence and types of MRPs and interventions in LT recipients with and without an outpatient pharmacy consultation by a clinical pharmacist as well as the satisfaction with information about medicines and medication adherence.
Ethics approval
A waiver was given by the Medical Ethics Committee of the Erasmus University Medical Center (MEC-2019-0784). Patient data were sampled and stored in accordance with privacy regulations.
Method
Study design and setting
We performed an observational cohort study at the Erasmus MC Transplant Institute, University Medical Center Rotterdam, the Netherlands. For our primary objective, we retrospectively collected data between July-December 2020. For the secondary objectives, we prospectively included a second cohort of LT recipients to participate in a questionnaire study in the period March-May 2021. Both cohorts consisted of adult LT recipients > 1 year after LT who were scheduled for an annual, multidisciplinary medical check-up at the outpatient clinic. A retro- and prospective cohort were used due to practical and feasibility reasons. The use of questionnaires to evaluate the satisfaction with information about medicines and medication adherence was not regularly done in our clinic. Therefore, the prospective part of this research was performed during a research internship. No differences in the treatment protocol occurred during both periods.
At the start of this study in July 2020, 746 LT recipients were in active follow up after transplantation at the Erasmus MC. Since 2018, a clinical pharmacist has an active role in the annual, multidisciplinary medical check-up of LT recipients by conducting MCs. Detailed information about the content of the MCs by the clinical pharmacist has been previously reported [12]. All check-ups are performed on two weekdays, with the clinical pharmacist only participating on one of these days. This has resulted in two cohorts: a cohort that did not receive a MC (non-MC cohort) and a cohort that did receive a MC (MC cohort). In the non-MC cohort more LT recipients are being seen since more hepatologists have outpatient visits on that day of the week. All findings during the check-ups were registered in the patients’ medical records for further follow-up.
Data collection
MRPs and interventions
For the analysis of the primary objective the following baseline characteristics were obtained from the patient medical record: age, gender, indication for liver transplantation, time after transplantation, information about re-transplantation, comorbidities, and number and type of drugs. MRPs and interventions in the non-MC cohort were identified by reviewing all information. This information included the patients’ medical history and laboratory results, such as electrolytes, renal function, and blood glucose levels. Medication reconciliation was performed in the MC cohort. MRPs and interventions identified by the clinical pharmacist as well as MRPs solved by the clinical pharmacist in the MC cohort were documented in the patients’ medical records.
Assessment of MRPs and interventions
For the non-MC cohort, MRPs were assessed by a pharmacological review based on all available information in the patients’ medical record after the LT recipients were seen by a hepatologist. The follow-up and corrections of the detected MRPs in the non-MC cohort was beyond the scope of this research. Two researchers independently identified these MRPs and interventions proposed by the hepatologist and categorized them into predefined categories using the Pharmaceutical Care Network Europe (PCNE) Classification V9.0 (full classification in Supplementary Table 1) [8]. Next, the classifications were compared and when dissensus occurred, both researchers reviewed their classifications and discussed these until consensus was reached.
For the MC cohort, the two researchers independently categorized the identified MRP and proposed interventions by the clinical pharmacist as registered in the patients’ medical record into the predefined categories using the PCNE Classification V9.0 [8]. Only one intervention could have been proposed for each identified MRP.
Satisfaction with Information about Medicines and the Medication Adherence
LT recipients in the second cohort were asked to fill out two questionnaires (translated and validated into Dutch) after their annual medical check-up: the Satisfaction with Information about Medicines (SIMS) and the Medication Adherence Reporting Scale (MARS-5) surveys [13, 14]. Besides, patients were asked to report their age, gender and highest reached educational level. The International Standard Classification of Education 2011 (ISCED 2011) was used to convert the Dutch educational system into an international one [15].
Assessment of the Satisfaction with Information about Medicines and the Medication Adherence
The SIMS assesses patients’ satisfaction with 17 items of information considered essential for safe and accurate self-management of medicines according to the recommendations of the Association of the British Pharmaceutical Industry (full survey in Section S1 in the Supplementary Appendix) [13, 14]. For each item, patients indicate if the information they have received is “too much,” “about right,” “too little,” “none received,” or “none needed.” Reports of “about right” and “none needed” are classified as satisfied and receive a score of 1. The remaining options are classified as dissatisfied and are scored as 0. The scores are summed up to obtain a satisfaction rating for the total scale ranging from 0 to 17. Higher summary scores indicate a higher degree of satisfaction with information received [16]. A score of ≥ 13 was interpreted as a satisfied patient and a number < 13 was interpreted as a dissatisfied patient. No threshold for satisfied versus dissatisfied was described in the literature. Therefore, an arbitrary threshold in which > 75% of the items of the questionnaire received a score of 1 was chosen by the researchers.
The MARS-5 compromises five short adherence statements (full survey is provided in Section S2 in the Supplementary Appendix) [14]. The MARS-5 survey was scored on a Likert-type scale ranging from “always”, “often”, “sometimes”, “rarely” to “never”. The point spread from 1 point for “always” to 5 points for “never”. A total adherence rate was obtained for each patient. A total of 25 could be achieved with higher scores indicating higher reported adherence.
Statistical analysis
No formal sample size calculation was performed. We included every patient seen for their annual check-up in the study period at the outpatient clinic in the analysis. Variables were described using counts (%) for nominal and ordinal variables and mean (standard deviation, SD) or median (inter-quartile range, IQR) for the continuous variables, depending on the shape of the distribution.
The primary and secondary endpoints were analyzed using Pearson’s Chi-square test or Fisher’s exact tests. The latter was used in the case of a low observed count (< 10) in at least one of the cohorts. For all statistical tests, a two-sided p-value of < 0.05 was considered to indicate statistical significance. Missing values < 5% were considered as missing completely at random.
Statistical analysis was performed using SPSS for Windows, version 25.0 (SPSS, Chicago, IL).
Results
Primary endpoint: prevalence of and interventions proposed for MRPs
Between 30/06/2020–31/12/2020 a total of 291 LT recipients had their annual, medical check-up; 197 in the non-MC cohort and 94 in the MC cohort. Two LT recipients in the MC cohort did not show up at their annual, medical check-up.
Table 1 shows the baseline clinical and demographical characteristics of the participants. LT recipients in the non-MC cohort had a significantly higher occurrence of renal disorder as comorbidity (p < 0.001). No significant differences were found in the number of drugs on the medication list during consultation during the annual check-up (p = 0.276).
Medication related problems
In total, 616 MRPs were identified: 368 in the non-MC cohort (median per patient, 1.0; IQR, 1.0–3.0) and 248 in the MC cohort (median per patient, 2.0; IQR, 1.0–4.0). Most LT recipients had at least one MRP, 173 (87.8%) in the non-MC cohort and 89 (94.7%) in the MC cohort.
Table 2 shows the prevalence and examples of the identified MRPs in both cohorts. In the MC cohort, significantly fewer MRPs as unnecessary drugs (17.3% versus 58.7%, p < 0.001), wrong drugs/suboptimal therapy (2.4% versus 9.5%, p < 0.001), untreated indication (2.8% versus 6.8%, p = 0.040), and too low dosed drugs (0.4% versus 6.3%, p < 0.001) were detected compared to the non-MC cohort. In the MC cohort significantly more MRPs as unintentional nonadherence (9.0% versus 0.0%, p < 0.001), problems in drug use (24.2% versus 1.6%, p < 0.001), questions regarding the drugs (4.0% versus 0.0%, p < 0,001), and other problems (28.2% versus 3.0%, p < 0.001) were detected compared to the non-MC cohort.
In the non-MC cohort significantly more patients used unnecessary drugs (72.1% versus 39.4%, p < 0.001) compared to the MC cohort and 16.5% of the patients in the non-MC cohort used suboptimal doses. The most prevalent unnecessary drugs used were proton pump inhibitors, opioids, benzodiazepines, vitamin D and calcium supplementation. Suboptimal doses were mostly found in patients with poorly controlled diabetes.
A total of 35 LT recipients in the MC cohort had their first MC with the clinical pharmacist, 55 had their second MC and 4 had their third MC. Patients having their first MC with the clinical pharmacist had the most MRPs. The number of MRPs for ADRs in patients having their second MC with the clinical pharmacist was reduced compared to patients having their first MC (16.2% versus 9.7%). The number of MRPs for unnecessary drugs, usage issues and discrepancies in the medication list in patients having their second MC did not differ compared to patients having their first MC.
Interventions proposed for MRPs
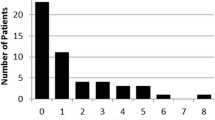
Figure 1 shows the interventions proposed for the MRPs. A total of 74 interventions were proposed by the hepatologist in the non-MC cohort (median, 0.0; IQR, 0.0–1.0; maximum 2) and 251 interventions were proposed by the clinical pharmacist in the MC cohort (median, 2.0; IQR, 1.0–4.0; maximum 10). Interventions in the non-MC cohort and MC cohort were carried out by a hepatologist. The most prevalent interventions by a hepatologist were starting a drug (28/74, 37.8%), changing a dose (24/74, 32.4%), and pausing or stopping a drug (15/74, 20.3%). The most prevalent interventions by the clinical pharmacist were adjusting the patient file (71/251, 28.3%), changing the instructions for use (42/251, 16.7%) and pausing or stopping a drug (36/251, 14.3%).
The clinical pharmacist resolved 251 MRPs of which 155 (61.8%) were accepted by the hepatologist and 46 (18.3%) were not accepted by the hepatologist. Examples of accepted interventions were: lowering the dose of magnesium hydroxide and stopping proton pump inhibitors due to the absence of an indication for the high dose and optimizing the dosing regimen. Examples of interventions not accepted were: stopping drugs prescribed by another physician due to the absence of an indication, optimizing the antihypertensive therapy according to the guidelines and changing to another class of laxatives because of taste complaints by the patient. Due to the need for follow-up, it is unknown if 11 (4.4%) interventions were accepted by the LT recipient and due to the nature of the MRP 39 (15.5%) interventions could not be followed up.
Secondary endpoints: Satisfaction with Information about Medicines and the Medication Adherence
A total of 132 LT recipients participated in the surveys: 84 in the non-MC cohort and 48 in the MC cohort. The completion rate was 80.5%.
Baseline characteristics of participants for the SIMS and MARS-5 surveys are shown in Table 3. The median age differed significantly between the two groups (54.0 years (IQR: 43.0–65.0) in the non-MC cohort versus 63.5 years (IQR: 54.0–68.0) in the MC cohort (p = 0.027)). The majority in both groups were men: 60.7% in the non-MC cohort and 64.4% in the MC cohort.
Satisfaction with Information about Medicines
Table 4 shows the overall satisfaction and factors associated with the satisfaction of LT recipients with the information about medicines in both cohorts. LT recipients in both cohorts are satisfied with the information about medicines with lower educated LT recipients less satisfied and higher educated LT recipients more satisfied. LT recipients aged < 55 and > 65 years appeared to be more satisfied in the MC cohort compared to the non-MC cohort. LT recipients in the MC cohort were more satisfied compared to the non-MC cohort (72,9% versus 64,3%, p = 0.309).
LT recipients in both cohorts were less satisfied with the information about the mechanism of action, ADRs and whether the medicine interferes with other drugs (supplementary Table 2).
Medication Adherence
LT recipients in both cohorts had a median MARS-5 score of 24.0 (IQR: 24.0–25.0), which indicates a high medication adherence in both cohorts.
Discussion
To our knowledge, this is the first study describing the impact of a clinical pharmacist by investigating the differences in MRPs in LT patients with and without an MC during an outpatient annual check-up. LT recipients in the MC cohort had significantly fewer MRPs as using of unnecessary drugs, wrong drugs/suboptimal therapy, and having dosing issues. In the MC cohort significantly more MRPs were identified as unintentional nonadherence, problems in drug use, questions regarding the drugs, and other problems. We demonstrated a high prevalence of MPRs in LT recipients in the outpatient setting with a median of 1 MRP in the non-MC cohort and 2 MRPs in the MC cohort. The most frequently reported MRPs in both cohorts were unnecessary drug use, problems in drug use and ADRs. Most LT recipients in both cohorts were satisfied with the information about medicines.
Our findings are in line with the results of Flamme-Obry et al. They showed that an interview with the clinical pharmacist at discharge could help to reduce MRPs in kidney transplant recipients [17]. Flamme-Orby found that their intervention resulted in fewer MRPs as interactions between drugs, ADRs and wrong usage of medicines. By introducing a medication consultation by a clinical pharmacist we resolved a substantial number of MRPs as the wrong usage of medicines. Furthermore, several studies showed comparable results to ours with regards to the most prevalent MRPs [10, 18]. The most prevalent MRPs in these studies were ADRs, nonadherence issues, and the use of unnecessary drugs. By detecting and preventing the use of unnecessary drugs the clinical pharmacist can contribute to relevant social issues as preventing for unnecessary healthcare costs and the sustainable use of medicines.
Most frequently proposed interventions by the clinical pharmacist in the MC cohort were instructions for use changed, drug paused or stopped and patient file adjusted. Cohen et al. showed that a pharmacist-driven medication reconciliation significantly reduced medication discrepancies [19]. Furthermore, another study by Ho et al., showed that pharmacists working as part of the multidisciplinary transplant team identified and resolved medication discrepancies and thereby improved the medication safety at a transplant clinic [20]. Interestingly, in both studies the average number of discrepancies per patient was higher compared to our study. This might been explained by the fact that in the Netherlands medication reconciliation is mandatory for every patient admitted to a hospital. The most frequently proposed interventions by the hepatologist in the non-MC cohort were the start/stop of a drug or the change of the dosage of a drug. This is a consequence of the organization of the Dutch healthcare system in which only doctors are allowed to initiate those interventions. Interventions not accepted by the hepatologist were mostly due to the fact that these drugs were prescribed by other prescribers or patients were not willing to change/stop their medication.
Most LT recipients in both cohorts were satisfied with the information about medicines. In the non-MC cohort, patients indicated that they were less satisfied with the information regarding the mechanism of action, ADRs and possible interactions. This might be explained by the fact that hepatologists focus less on educating patients about their medicines and ADRs. These findings are in line with Klewitz et al. who found a high prevalence of dissatisfaction with information about medication, specifically ADRs, in kidney transplant recipients [16]. A study evaluating the SIMS in patients using anticancer agents also showed comparable results to our non-MC cohort with patients dissatisfied with the information concerning the mechanism of action of drugs, the risk of ADRs, and the interference with other drugs [21]. Based on the results in this study, educating patients about their medication, ADRs and dosing regimens is not commonly done but is important to prevent for MRPs.
Strengths of our study are the real-life clinical setting and the fact that we included a representative part, almost 50%, of the LT recipients being seen at the outpatient clinic. A limitation of our study is the fact that in the non-MC cohort, MRPs and interventions were detected using the patients’ medical records, including the patients’ medication list, laboratory results and medical history. The clinical pharmacist did not attend the consultations by the hepatologist. Whereas patients in the MC cohort had an actual MC with the clinical pharmacist. As a consequence, other types of and less MRPs were detected in the non-MC cohort since less information was available. Another limitation is the fact that we did not investigate the impact of the MRPs in relation to potential harm and clinical outcomes. Further research is needed to study the cost-effectiveness of the MCs carried out by a clinical pharmacist. In addition, evaluating the responsibilities and mandate of a clinical pharmacist to resolve MRPs caused by drugs prescribed by different physicians is needed to optimize the medication safety in patients with multiple comorbidities.
Conclusion
LT recipients in the MC cohort had significantly fewer MRPs as the usage of unnecessary drugs, wrong drugs/suboptimal therapy, and having dosing issues. Over 70% of the patients in the non-MC cohort were using unnecessary drugs. LT recipients in both cohorts are satisfied with the information about medicines. Since clinical pharmacists bring different perspectives to post-transplant care, including a clinical pharmacist in the multidisciplinary transplant team has an added value for improving the pharmaceutical care and optimizing medication safety in these patients.
References
Moon D, Lee S. Liver transplantation. Gut Liver. 2009;3:145–65.
Neuberger JM, Bechstein WO, Kuypers DR, et al. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation. 2017;101:1–56.
Vranian SC, Covert KL, Madis CR, et al. Assessment of risk factors for increased resource utilization in kidney transplantation. J Surg Res. 2018;222:195–202.
De Geest S, Burkhalter H, De Bleser L, et al. Immunosuppressive drugs and non-adherence in transplantation. J Ren Nurs. 2010;2:58–63.
Nevins TE, Nickerson PW, Dew MA. Understanding Medication Nonadherence after Kidney Transplant. J Am Soc Nephrol. 2017;28:2290–301.
Moreno S, Berenguer M. Post-liver transplantation medical complications. Ann Hepatol. 2006;5:77–85.
Stemer G, Lemmens-Gruber R. Clinical pharmacy services and solid organ transplantation: a literature review. Pharm World Sci. 2010;32:7–18.
Pharmaceutical Care Network Europe. Classification for Drug related problems: the PCNE Classification V 9.0: Pharmaceutical Care Network Europe. [Internet]. Available from: https://www.pcne.org/upload/files/334_PCNE_classification_V9-0.pdf. Accessed 09.03.2021.
Leendertse AJ, Egberts AC, Stoker LJ, et al. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–6.
Repp KL, Hayes C, Woods TM, et al. Drug-Related Problems and Hospital Admissions in Cardiac Transplant Recipients. Ann Pharmacother. 2012;46:1299–307.
Stemer G, Lemmens-Gruber R. Clinical pharmacy services and solid organ transplantation: a literature review. Pharm World Sci. 2010;32:7–18.
Mulder MB, Borgsteede SD, Murad SD, et al. Medication-Related Problems in Liver Transplant Recipients in the Outpatient Setting: A Dutch Cohort Study. Front Pharmacol. 2021;12:637090.
Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Qual Health Care. 2001;10:135–40.
Chan AHY, Horne R, Hankins M, et al. The Medication Adherence Report Scale: A measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86:1281–8.
Education in numbers. Assignment of Dutch educational programs (ISCED). Available from: https://www.onderwijsincijfers.nl/kengetallen/internationaal/toedeling-nederlandse-onderwijsprogrammas-isced. Accessed 21.06.2021.
Klewitz F, Nöhre M, Bauer-Hohmann M, et al. Information Needs of Patients About Immunosuppressive Medication in a German Kidney Transplant Sample: Prevalence and Correlates. Front Psychiatry. 2019;10:444.
Flamme-Obry F, Belaiche S, Hazzan M, et al. Clinical pharmacist and medication reconciliation in kidney transplantation. Nephrol Ther. 2018;14:91–8.
Taber DJ, Pilch NA, Bratton CF, et al. Medication errors and adverse drug events in kidney transplant recipients: incidence, risk factors, and clinical outcomes. Pharmacotherapy. 2012;32:1053–60.
Cohen EA, McKimmy D, Cerilli A, et al. A Pharmacist-Driven Intervention Designed to Improve Medication Accuracy in the Outpatient Kidney Transplant Setting. Drug Healthc Patient Saf. 2020; 12: 229–235.
Ho L, Akada K, Messner H, et al. Pharmacist’s Role in Improving Medication Safety for Patients in an Allogeneic Hematopoietic Cell Transplant Ambulatory Clinic. Can J Hosp Pharm. 2013; 66: 110–117.
Boons CCLM, Timmers L, Schoor NM, et al. Patient satisfaction with information on oral anticancer agent use. Cancer Med. 2018;7:219–28.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mulder, M.B., Doga, B., Borgsteede, S.D. et al. Evaluation of medication-related problems in liver transplant recipients with and without an outpatient medication consultation by a clinical pharmacist: a cohort study. Int J Clin Pharm 44, 1114–1122 (2022). https://doi.org/10.1007/s11096-022-01423-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01423-6