Abstract
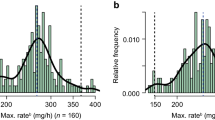
Background Infusion-related reactions (IRRs) during rituximab administration are occasionally severe and remain problematic in oncology practice. Aim To establish a safer, risk-stratified rituximab protocol for patients with B-cell lymphoma. Method We stratified patients into low-, moderate-, and high-risk groups according to the number of risk factors for IRRs, specifically, low-grade histology and bulky tumors (> 10 cm): Then, the administrating schedule of rituximab (375 mg/m2, diluted in 1 mg/mL concentration) was individualized. For the first rituximab cycle, the low- and moderate-risk groups underwent conventional infusion #1 (25–200 mg/h, ~4.3 h), and the high-risk group underwent long infusion (25–100 mg/h, 6.8 h). Patients in the low-, moderate-, and high-risk groups without IRRs in the first cycle underwent short infusion (100–400 mg/h, 2.3 h), conventional infusion #2 (100–200 mg/h, 3.5 h), and conventional infusion #1, respectively. Patients with IRRs in the first cycle received a second rituximab cycle with the same schedule as the first cycle. The procedure for the third cycle was at the attending physician’s discretion. Results Among 81 patients, the overall incidence of IRRs was 28%. IRR incidences in the low- (n = 39), moderate- (n = 35), and high-risk groups (n = 7) were 31%, 20%, and 57%, respectively. All IRRs were grade ≤ 2. The overall conversion rate to short infusion in the third cycle was 54%, without any IRRs. Conclusions Our step-by-step rituximab protocol demonstrated a fewer incidence of severe IRRs among B-cell lymphoma patients receiving rituximab.




Similar content being viewed by others
References
Salles G, Barrett M, Foà R, Maurer J, O’Brien S, Valente N et al. Rituximab in B-Cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–73.
Paul F, Cartron G. Infusion-related reactions to rituximab: frequency, mechanisms and predictors. Expert Rev Clin Immunol 2019;15:383–9.
Jung JW, Kang HR, Lee SH, Cho SH. The incidence and risk factors of infusion-related reactions to rituximab for treating B cell malignancies in a single tertiary hospital. Oncologist 2014;86:127–34.
D’Arena G, Simeon V, Laurenti L, Cimminiello M, Innocenti I, Gilio M, et al. Adverse drug reactions after intravenous rituximab infusion are more common in hematologic malignancies than in autoimmune disorders and can be predicted by the combination of few clinical and laboratory parameters: results from a retrospective, multicent. Leuk Lymphoma 2017;58:2633–41.
Schwartzberg LS, Stepanski EJ, Walker MS, Mathias S, Houts AC, Fortner BV. Implications of IV monoclonal antibody infusion reaction for the patient, caregiver, and practice: results of a multicenter study. Support Care Cancer. 2009;17:91–8.
Schwartzberg LS, Stepanski EJ, Fortner BV, Houts AC. Retrospective chart review of severe infusion reactions with rituximab, cetuximab, and bevacizumab in community oncology practices: assessment of clinical consequences. Support Care Cancer 2008;16:393–8.
Vogel H W. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs 2010;14:E10–21.
Hayama T, Miura K, Uchiike A, Nakagawa M, Tsutsumi D, Sakagami M, et al. A clinical prediction model for infusion-related reactions to rituximab in patients with B cell lymphomas. Int J Clin Pharm 2017;39:380–5.
Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 2008;13:725–32.
Common terminology criteria for adverse events. 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/. Accessed on 10 Sept 2021.
Jung JW, Kang HR, Lee SH, Cho SH. The incidence and risk factors of infusion-related reactions to rituximab for treating B cell malignancies in a single tertiary hospital. Oncology. 2014;86:127–134.
Atmar J. Review of the safety and feasibility of rapid infusion of rituximab. J Oncol Pract 2010;6:91–3.
Provencio M, Cerdeira S, Bonilla F, Sánchez A, España P. Rapid-infusion rituximab in lymphoma treatment. Ann Oncol 2006;17:1027–8.
Lang DS, Keefe DM, Schultz T, Pearson A. Predictors of acute adverse events from rapid rituximab infusion. Support Care Cancer 2013;21:2315–20.
Dakhil S, Hermann R, Schreeder MT, Gregory SA, Monte M, Windsor KS, et al. Phase III safety study of rituximab administered as a 90-minute infusion in patients with previously untreated diffuse large B-cell and follicular lymphoma. Leuk Lymphoma 2014;55:2335–40.
Courville J, Nastoupil L, Kaila N, Kelton J, Zhang J, Alcasid A, et al. Factors influencing infusion-related reactions following dosing of reference rituximab and PF-05280586, a rituximab biosimilar. BioDrugs. 2021;35:459–68.
Sharman JP, Liberati AM, Ishizawa K, Khan T, Robbins J, Alcasid A, et al. A randomized, double-blind, efficacy and safety study of PF-05280586 (a rituximab biosimilar) compared with rituximab reference product (MabThera®) in subjects with previously untreated CD20-positive, low-tumor-burden follicular lymphoma (LTB-FL). BioDrugs 2020;34:171–81.
Acknowledgements
The authors thank Mr. I. Odagiri, Mr. T. Yamauchi, Dr. H. Takahashi, Dr. M. Nakagawa, Dr. Y. Uchino, Dr. K. Iizuka, Dr. T. Hamada, and Dr. N. Iriyama for their enormous contributions to this work.
Funding
This study was not funded by any third parties.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K. M. and Y. H. received speaker fees from Chugai and Kyowa-Kirin, which manufactures rituximab and its biosimilar products in Japan. The other authors have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was registered in UMIN-CTR (https://www.umin.ac.jp/english/) on 19th April 2018 (Registration ID: UMIN000032309).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsutsumi, D., Hayama, T., Miura, K. et al. A novel rituximab administration protocol to minimize infusion-related adverse reactions in patients with B-cell lymphoma. Int J Clin Pharm 44, 366–373 (2022). https://doi.org/10.1007/s11096-021-01348-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-021-01348-6