Abstract
Background Chronic drug therapy may impact recurrence and survival of patients with bladder cancer and thus be of concern regarding drug choice and treatment decisions. Currently, data are conflicting for some drug classes and missing for others. Objective To analyze the impact of common non-oncologic chronic drug intake on survival in patients with bladder cancer and radical cystectomy. Setting. Patients with bladder cancer and radical cystectomy (2004–2018) at the University Hospital Munich. Method Data from an established internal database with patients with bladder cancer and radical cystectomy were included in a retrospective study. Drug therapy at the time of radical cystectomy and survival data were assessed and follow-up performed 3 months after radical cystectomy and yearly until death or present. Impact on survival was analyzed for antihypertensive, antidiabetic, anti-gout, antithrombotic drugs and statins, using the Kaplan–Meier method, log-rank test and Cox-regression models. Main outcome measure Recurrence free survival, cancer specific survival and overall survival for users versus non-users of predefined drug classes. Results Medication and survival data were available in 972 patients. Median follow-up time was 22 months (IQR 7–61). In the univariate analysis, a significant negative impact among users on recurrence free survival (n = 93; p = 0.038), cancer specific survival (n = 116; p < 0.001) and overall survival (n = 116; p < 0.001) was found for calcium-channel blockers, whereas angiotensin-receptor-blockers negatively influenced overall survival (n = 96; p = 0.020), but not recurrence free survival (n = 73; p = 0.696) and cancer specific survival (n = 96; p = 0.406). No effect of angiotensin-receptor-blockers and calcium-channel blockers was seen in the multivariate analysis. None of the other studied drugs had an impact on survival. Conclusion There was no impact on bladder cancer recurrence and survival for any of the analyzed drugs. Considering our results and the controverse findings in the literature, there is currently no evidence to withhold indicated drugs or choose specific drug classes among the evaluated non-oncologic chronic drug therapies. Thus, prospective studies are required for further insight. Trail registration This is part of the trial DRKS00017080, registered 11.10.2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact on practice
-
The survival of bladder cancer patients after radical cystectomy is not altered by the chronic use of antihypertensives, anticoagulants, antidiabetic drugs, statins or allopurinol. However, data are still limited and prospective studies are urgently needed.
-
At present, there is no evidence to withhold any indicated chronic drug therapy for patients with bladder cancer and no recommendation for the use of a specific drug class of e.g. antihypertensives or antidiabetics can be made.
-
Optimal treatment for the underlying comorbidities is suggested to be the most important goal irrespective of the drug selection and patients should be encouraged to continue their chronic co-medication and prescription of indicated treatment for chronic diseases should be continued.
Introduction
Urothelial carcinoma of the urinary bladder (UCB) is the 11th most common cancer worldwide and is characterized by high mortality and recurrence rate [1]. Radical cystectomy (RC) represents the gold standard of intervention for localized muscle-invasive (MIBC) and recurring high-risk non-muscle-invasive bladder cancer (NMIBC) [1]. When first diagnosed, most patients are 70 years or older at which age more than 90% of the patients regularly take drugs [2]. The most often diagnosed comorbidities in cancer patients, e.g. hypertension or diabetes, demand chronic drug therapy [3]. A possible impact of chronic drugs on the risk of UCB development has been discussed for several substances, a prominent example being the thiazolidinedione pioglitazone [4]. While some studies are available on the impact of drugs on the risk of UCB development [5], data regarding an impact on recurrence and survival are still limited. For instance, conflicting results have been found for first-line antihypertensives like angiotensin-converting-enzyme inhibitors (ACEI), angiotensin-receptor-blockers (ARB) and calcium-channel blockers (CCB) [6,7,8,9,10,11,12]. Although the precise mechanism remains unclear, the renin–angiotensin–aldosterone system potentially affects carcinogenesis and tumor progression [13]. Of interest, a positive impact of the oral antidiabetic drug metformin has been described in a meta-analysis, but contradicting results have been reported by more recent studies [14, 15]. There are no or very limited data on the impact of other chronically administered drugs, such as additional oral antidiabetics, anticoagulants or anti-gout drugs. On the other hand, in vitro data or biological modelling systems concerning other cancer entities point towards a possible effect of some drug classes on UCB recurrence and progression [16,17,18]. Low dose ASA is frequently used for the prevention of thromboembolic events in elderly patients. Antineoplastic effects have been discussed for several cancer entities, e.g. colorectal, breast and gastric cancer [19]. Vitamin-K-antagonists are widely used for prophylaxis and therapy for thromboembolic events e.g. in patients with atrial fibrillation or deep vein thrombosis. While studies have found a lower risk for overall cancer incidence in chronic warfarin users [17], no protective effect but a non-significant risk elevation by 27% was described for warfarin on UCB development [20]. Whereas the anti-gout drug allopurinol has been linked to an increased overall cancer incidence, its impact on UCB development remains obscure [21].
While a positive impact of chronically administered non-oncologic drug classes might allow to tailor drug therapy according to patients´ risks, e.g. in the choice of antihypertensive agents, a negative impact could present a therapeutic dilemma when drug therapy of comorbidities is necessary. Thus, more evidence is needed regarding the impact of chronic drug intake on UCB recurrence and survival.
Aim of the study
To analyze the impact of drug therapy with antihypertensives, antidiabetics, antithrombotics, statins and the anti-gout drug allopurinol on recurrence rate and survival in a retrospective cohort of UCB patients undergoing radical cystectomy.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki. Approval was obtained by the ethics committee of the University Hospital Munich (18–427).
Method
A retrospective observational study was performed at a tertiary teaching hospital using data from an established database of UCB patients at the department of urology of the University Hospital LMU Munich [22]. The database includes UCB patients, who underwent RC between 2004 and 2018.
Data collection
The following data was collected from the UCB patient database: age, sex, pathological staging (TNM), grading (G), surgical margin (R) and follow-up data on oncologic outcome. The first follow-up was performed three months after RC and continued once yearly with validated questionnaires sent by regular mail, as described previously [22]. Patients were followed-up until present date or until death and recurrence free survival (RFS), cancer specific survival (CSS) and overall survival (OS) were documented [22]. Cause of death was defined by the treating physician or a death certificate. Bladder specimens after RC were examined according to standard histopathological procedures by urogenital pathologists at the Department of Pathology. Tumor staging and grading was undertaken according to the TNM classification and World Health Organization grading criteria [1]. Data on drug therapy from the day of RC were taken from patients` drug charts and added to the database. A pharmacist checked the medication data for completeness and plausibility using the hospitals electronic patient information system (SAP-i.s.h.med, Cerner Corporation, North Kansas City, USA). No additional data on continued or additional drug intake were assessed at follow-ups.
Analyzed drugs and drug classes
Previously studied non-oncologic drugs and drug classes used for chronic therapy were selected based on their potential effect on UCB development, recurrence or known impact on other cancer entities [6, 9, 10, 12, 15, 17, 23,24,25]. The following drug classes defined by anatomical therapeutical chemical code (ATC) were included: antihypertensives (ACEI (C09AA), ARB (C09CA), beta blockers (C07AB), CCB (C08)), drugs targeting blood coagulation (acetylsalicylic acid (ASA) (B01AC), vitamin-K-antagonists (B01AA), direct acting oral anticoagulants (DOAC) (B01AF)), antidiabetic drugs (metformin (A10BA02), sulfonylureas (A10BB), thiazolidinediones (A10BG), dipeptidylpeptidase 4 (DPP4)-Inhibitors (A10BH), insulin (A10A)) and miscellaneous (statins (C10AA), allopurinol (M04AA01)). Based on the medication review, patients were classified as user or non-user for every drug class included.
Statistical analysis
Primary endpoints were RFS and CSS, secondary endpoint was OS. Survival curves were generated by the Kaplan–Meier method and compared by log-rank tests. Hazard ratios (HR) were estimated by Cox analysis with a 95% confidence interval. An univariate analysis was performed for the association between survival and drug intake (user/non-user). If univariate analysis showed a significant result for a drug class, a subsequent multivariate analysis (Cox regression) including other factors predicting survival (age, sex, tumor staging and grading) was performed. A two-sided p-value ≤ 0.05 was considered statistically significant. Continuous data are presented as median and interquartile range (IQR). Statistical analyses were performed and figures created with Microsoft Excel®2016 (Seattle, WA, USA) and MedCalc®17 software (MedCalc, Ostend, Belgium).
Results
Patient characteristics
Overall, for 972 patients with UCB undergoing RC, follow-up and medication data were available and OS and CSS were analyzed. In addition, for 859 patients, data on recurrence were available and RFS analysis was performed. The median follow-up time was 22 months (IQR 7–61 months, max. 170 months). Patient characteristics are presented in Table 1.
Influence of age, sex, tumor staging and grading on survival of patients with UCB
As seen in Table 3, T-stage, N-status and presence of metastasis (TNM), R-status and age each had an independent influence on RFS, CSS and OS in the multivariate analysis. No independent impact on RFS, CSS and OS was found for tumor grade (G) and sex.
Influence of antihypertensive drugs on recurrence and survival of patients with UCB
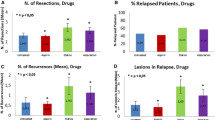
Regarding antihypertensives, 286 patients took beta blockers, 274 ACEI, 96 ARB and 116 patients CCB (Table 2). Comparing users with non-users in an univariate analysis, no impact on RFS, CSS and OS was found for ACEI and beta blockers (Table 2). Of note, CCB had a statistically significant negative impact on RFS, CSS and OS (Fig. 1). The median survival of non-users versus users was 145 compared to 30 months for RFS, 119 versus 45 months for CSS and 63 versus 24 months for OS. ARB had a significant negative impact on OS, but no impact on RFS (Fig. 2). The median OS of non-users versus users was 59 compared to 38 months. To test whether the drugs, which were shown to influence survival in patients with UCB in the univariate analysis, have an impact on RFS, CCS and OS in combination with other factors predicting survival in these patients, we performed a multivariate Cox regression analysis. ARB and CCB, which had a negative impact on several parameters in the univariate analysis, did not show any independent impact on RFS, CSS or OS in the multivariate analysis (Table 3). Antihypertensives without significant result in the univariate analysis were not considered in the multivariate analysis.
Influence of antidiabetic drugs on recurrence and survival of patients with UCB
In our cohort, 57 patients were treated with the oral antidiabetic metformin, 25 with insulin, 21 with sulfonylureas, 16 with DDP4-inhibitors and 6 with thiazolidinediones (Table 2). No association between the intake of insulin, metformin, sulfonylureas and DDP4-inhibitors and survival (RFS, CCS and OS) was found (Table 2) in comparison to non-users in the univariate analysis. The small number of patients treated with thiazolidinediones (n = 6) precluded a statistical analysis for this drug class. Since antidiabetics showed no effect in the univariate analysis, they were not included in the multivariate analysis.
Influence of other frequently used drugs on recurrence and survival of patients with UCB
No association between the anticoagulants ASA, vitamin-K-antagonists and DOACs and RFS, CCS and OS was found (Table 2). Additionally, for the cholesterol lowering statins as well as for the anti-gout drug allopurinol, no correlation to RFS, CCS or OS was found. With no significant impact in univariate analysis, these drugs were not included in multivariate analysis.
Discussion
We retrospectively analyzed the impact of various common non-oncologic drugs on RFS, CSS and OS in UCB patients undergoing RC. In the multivariate analysis, no effect was found for intake of several classes of first-line antihypertensives, statins, the anti-gout drug allopurinol and antithrombotic drugs.
ACEI and ARB are first-line drugs for the treatment of hypertension according to national and international guidelines [26, 27]. Previous retrospective studies have shown improved outcomes for patients with NMIBC and MIBC taking ACEI or ARB. However, studies were small with 40 to 143 patients [6,7,8, 11]. In our study, we were able to analyze a higher number of patients with 274 on ACEI and 96 on ARB. In addition, the aforementioned studies mostly did not analyze ACEI and ARB separately, thus possibly masking differences in their impact on cancer recurrence and survival. Some evidence exists that this might be of importance, although the mechanism is unknown. A study evaluating the risk for urinary tract cancer development enrolling over 30,000 patients found no effect for ACEI, while ARB increased the risk [10]. Recently, a Finnish retrospective study evaluating the impact of antihypertensives on survival after bladder cancer diagnosis found no effect for ACEI, but an improved survival for ARB [9]. Of note, the positive ARB effect was only significant in the multivariate analysis for men, but not women. Our results agree with the Finnish findings concerning ACEI, but not for ARB (Table 2). Unfortunately, we were unable to differentiate the effect of ARB intake by sex, since only 16 of the 73 patients were female (data not shown). In contrast to our study focusing on patients with RC, the Finnish evaluation included all UCB patients, independent of the chosen treatment.
While beta blockers represented the most common antihypertensives in our analysis, no impact on RFS, CSS or OS was found. This is in line with findings by Dal Moro et al. (20 patients) and Santala et al. (500 patients) [8, 9].
For the antihypertensive drug class CCB, less data on the risk for UCB progression is available so far. In our study, use of CCB did have a negative impact on RFS, CSS and OS in the univariate analysis, while no effect was seen in the multivariate analysis. In the study by Dal Moro et al. only 14 patients received CCB and there was no significant effect on UCB recurrence [8].
Taken together, there are still conflicting results concerning a possible impact of different classes of antihypertensives on recurrence and survival in UCB patients but in our opinion, these effects may be neglected. For UCB development, hypertension has been identified as a risk factor (32% risk increase) with the effect being statistically significant in women and non-significant in men [28]. Dal Moro et al. found arterial hypertension to be a risk factor for UCB recurrence [8]. Thus, it has been discussed that appropriate treatment of hypertension is important, regardless of the antihypertensive drug class [9]. As a limitation of our study we were unable to include clinical outcome of the antihypertensive treatment in our analysis due to the retrospective design.
Low dose ASA was associated with an improved 5-year CSS and OS in a study including 1061 UCB patients with RC. In a multivariate analysis, ASA use was associated with lower cancer specific mortality and all-cause mortality [25]. In our study, including 246 ASA users, we could not confirm these findings. However, a possible trend towards an improved outcome for OS could be seen (p = 0.069, Table 2). Interestingly, Pastore et al. found a decrease in UCB recurrence after transurethral resection of the bladder for patients taking low dose ASA for at least two years, although the effect was no longer present with additional statin therapy [12]. Thus, additional diseases and co-medications may alter or mask an effect of low-dose ASA on UCB recurrence.
Of note, our study is the first analyzing the impact of vitamin-K-antagonist use on recurrence and outcome of UCB. Among the 36 patients taking phenprocoumon, we did not find an impact on UCB outcome.
To our knowledge, there are no studies available concerning a potential impact of DOAC on UCB outcome. No impact on recurrence and survival was found for the 23 patients in our cohort. Nevertheless, we expect that a rising number of UCB patients will take DOAC, as the risk for venous thrombosis after RC is high and DOAC are increasingly used for prophylaxis of cancer associated thrombosis [29]. Thus, more data on this issue can be expected in the near future.
In our study, insulin and oral antidiabetic drugs, except thiazolidinediones, did not show any impact on recurrence or survival in UCB patients. Pioglitazone, the only currently available thiazolidinedione in Germany, has been broadly discussed for an increased risk of bladder cancer development [4]. The facts are less clear for other oral antidiabetics. Diabetes without intake of the biguanide metformin has been described as a risk factor for recurrence and survival in patients with UCB [30]. A meta-analysis summarizing nine retrospective studies found a positive impact of metformin on RFS and CSS, but not OS [14]. Currently, a phase-II-study is investigating oral metformin in patients with NMIBC [31]. In contrast, a more recent population-based cohort study found no impact of metformin intake on CSS in UCB patients in a multivariate analysis [15]. In addition, the authors describe a negative impact for the sulfonylurea glyburide and no effect for other oral antidiabetics and insulin [15]. However, a lack of effect on UCB recurrence and survival for oral antidiabetic drugs and insulin was also described by others [32]. We assume that, like in hypertension, the adequate therapy of diabetes is the goal, which might reduce recurrence and improve survival in UCB patients.
Concerning allopurinol, a study from Taiwan found an increased overall cancer incidence for users, but no effect on UCB development [18]. To our knowledge, our study is the first analyzing the survival of UCB patients using allopurinol. No effect was found on recurrence and outcome in our cohort of 67 allopurinol users.
In coherence with a previous study [33], no effect on UCB recurrence and survival was seen for statins in our cohort. Conflicting results have been reported by others [12, 34, 35]. A direct and immediate anticancer effect of statins, described for several cancer entities in observational studies, has recently been questioned and explained as merely based on selection and immortal-time bias [36].
Considering the results of our study and the, in many aspects controversial, findings of other evaluations, a possible impact of chronic non-oncologic drug therapy on recurrence and survival in UCB patients seems unlikely. Even for drugs or drug classes with the most published data like metformin and antihypertensives, the evidence is conflicting. Several reasons might account for this. First, differences in the analyzed patient cohorts, time of observation and country-specific therapy guidelines may play a role. Second, the small number of patients included in some analyses might lead to statistical correct, but clinical misguiding results. In addition, as seen for some antihypertensive drug classes, gender effects may play a role, which have not been studied yet. Most important, all studies reporting on drug effects on UCB recurrence and survival so far, including the study presented here, are of a retrospective design. Finally, the duration and dosage of drug intake was not considered in our study, as is the case with most studies. Thus, prospective studies with adequate cohort sizes, following patients for an appropriate time and collecting data regarding drug exposure (duration and dosage) are urgently needed to gain more reliable data on the impact of chronic drugs on UCB recurrence and survival.
Conclusion
Considering the results of our and previous studies, there is currently no reason to withhold an indicated chronic drug therapy with antihypertensives, anticoagulants, statins, allopurinol or antidiabetics in patients with UCB and RC. However, data on many drugs and drug classes are still limited and the available evidence is based on small numbers and retrospective data. Despite these limitations, adequate treatment of concomitant diseases like hypertension and diabetes mellitus might be an important point for survival in UCB patients. Given the evidence available so far, no recommendation for a specific drug class of antihypertensives or antidiabetics is possible today.
References
Babjuk M BM, Compérat E, et al. EAU guideline: non-muscle-invasive bladder cancer. presented at the EAU Annual Congress Barcelona 2019.
Knopf H, Grams D. Medication use of adults in Germany: results of the German health interview and examination survey for adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5–6):868–77.
Roy S, Vallepu S, Barrios C, et al. Comparison of comorbid conditions between cancer survivors and age-matched patients without cancer. J Clin Med Res. 2018;10(12):911–9.
Tang H, Shi W, Fu S, et al. Pioglitazone and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2018;7(4):1070–80.
Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784–95.
Blute ML Jr, Rushmer TJ, Shi F, et al. Renin-Angiotensin inhibitors decrease recurrence after transurethral resection of bladder tumor in patients with nonmuscle invasive bladder cancer. J Urol. 2015;194(5):1214–9.
Yuge K, Miyajima A, Tanaka N, et al. Prognostic value of renin-angiotensin system blockade in non-muscle-invasive bladder cancer. Ann Surg Oncol. 2012;19(12):3987–93.
Dal Moro F, Bovo A, Crestani A, et al. Effect of hypertension on outcomes of high-risk patients after BCG-treated bladder cancer: a single-institution long follow-up cohort study. Med (Baltimore). 2015;94(9):e589.
Santala EEE, Kotsar A, Veitonmaki T, et al. Risk of urothelial cancer death among people using antihypertensive drugs-a cohort study from Finland. Scand J Urol. 2019;53(4):185–92.
Chuang YW, Yu MC, Huang ST, et al. Spironolactone and the risk of urinary tract cancer in patients with hypertension: a nationwide population-based retrospective case-control study. J Hypertens. 2017;35(1):170–7.
Yoshida T, Kinoshita H, Fukui K, et al. prognostic impact of renin-angiotensin inhibitors in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. 2017;24(3):823–31.
Pastore A, Palleschi G, Fuschi A, et al. Can daily intake of aspirin and/or statins influence the behavior of non-muscle invasive bladder cancer? A retrospective study on a cohort of patients undergoing transurethral bladder resection. BMC Cancer. 2015;15:120.
Ishikane S, Takahashi-Yanaga F. The role of angiotensin II in cancer metastasis: potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem Pharmacol. 2018;151:96–103.
Hu J, Chen JB, Cui Y, et al. Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(30):e11596.
Richard PO, Ahmad AE, Bashir S, et al. Impact of oral hypoglycemic agents on mortality among diabetic patients with non-muscle-invasive bladder cancer: a populationbased analysis. Can Urol Assoc J. 2018;12(6):203–10.
Wen W, Gong J, Wu P, et al. Mutations in gliclazide-associated genes may predict poor bladder cancer prognosis. FEBS Open Bio. 2019;9(3):457–67.
Haaland GS, Falk RS, Straume O, et al. Association of Warfarin use with lower overall cancer incidence among patients older than 50 years. JAMA Intern Med. 2017;177(12):1774–80.
Yang HC, Nguyen PAA, Islam M, et al. Gout drugs use and risk of cancer: a case-control study. Joint Bone Spine. 2018;85(6):747–53.
Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–27.
Blumentals WA, Foulis PR, Schwartz SW, et al. Does warfarin therapy influence the risk of bladder cancer? Thromb Haemost. 2004;91(4):801–5.
Chen CJ, Hsieh MC, Liao WT, et al. Allopurinol and the incidence of bladder cancer: a Taiwan national retrospective cohort study. Eur J Cancer Prev. 2016;25(3):216–23.
Grimm T, Buchner A, Schneevoigt B, et al. Impact of preoperative hemoglobin and CRP levels on cancer-specific survival in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: results of a single-center study. World J Urol. 2016;34(5):703–8.
Shih HJKM, Tsai PS, et al. Long-term allopurinol use decreases the risk of prostate cancer in patients with gout: a population-based study. Prostate Cancer Prostatic Dis. 2017;20(3):328–33.
Shih C, Hotaling JM, Wright JL, et al. Long-term NSAID use and incident urothelial cell carcinoma in the VITamins and Lifestyle (VITAL) study. Urol Oncol. 2013;31(8):1689–95.
Lyon TD, Frank I, Shah PH, et al. The association of aspirin use with survival following radical cystectomy. J Urol. 2018;200(5):1014–21.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–248.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
Kok VC, Zhang HW, Lin CT, et al. Positive association between hypertension and urinary bladder cancer: epidemiologic evidence involving 79,236 propensity score-matched individuals. Ups J Med Sci. 2018;123(2):109–15.
Klaassen Z, Arora K, Goldberg H, et al. extended venous thromboembolism prophylaxis after radical cystectomy: a call for adherence to current guidelines. J Urol. 2018;199(4):906–14.
Wang Q, Zhang T, Wu J, et al. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China. BMC Urol. 2019;19(1):24.
Molenaar RJ, van Hattum JW, Brummelhuis IS, et al. Study protocol of a phase II clinical trial of oral metformin for the intravesical treatment of non-muscle invasive bladder cancer. BMC Cancer. 2019;19(1):1133.
Nayan M, Bhindi B, Yu JL, et al. The effect of metformin on cancer-specific survival outcomes in diabetic patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Urol Oncol. 2015;33(9):38 e67-13.
Crivelli JJ, Xylinas E, Kluth LA, et al. Effect of statin use on outcomes of non-muscle-invasive bladder cancer. BJU Int. 2013;112(2):E4-12.
da Silva RD, Xylinas E, Kluth L, et al. Impact of statin use on oncologic outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy. J Urol. 2013;190(2):487–92.
Richard PO, Ahmad AE, Bashir S, et al. Effect of statins as a secondary chemopreventive agent among individuals with non-muscle-invasive bladder cancer: a population-based analysis. Urol Oncol. 2017;35(6):342–8.
Emilsson L, Garcia-Albeniz X, Logan RW, et al. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4(1):63–70.
Acknowledgements
This work was supported by the interprofessional doctoral programme Clinical Pharmacy, LMU Munich. We thank Dr. Ute Amann for advice on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Consent to participate
Not applicable due to the retrospective study design.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haimerl, L., Strobach, D., Mannell, H. et al. Retrospective evaluation of the impact of non-oncologic chronic drug therapy on the survival in patients with bladder cancer. Int J Clin Pharm 44, 339–347 (2022). https://doi.org/10.1007/s11096-021-01343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-021-01343-x