Abstract
Background
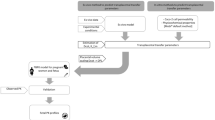
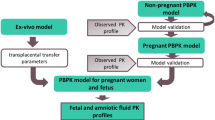
Evaluating drug transplacental clearance is vital for forecasting fetal drug exposure. Ex vivo human placenta perfusion experiments are the most suitable approach for this assessment. Various in silico methods are also proposed. This study aims to compare these prediction methods for drug transplacental clearance, focusing on the large molecular weight drug vancomycin (1449.3 g/mol), using maternal–fetal physiologically based pharmacokinetic (m-f PBPK) modeling.
Methods
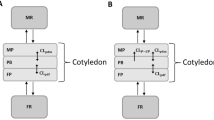
Ex vivo human placenta perfusion experiments, in silico approaches using intestinal permeability as a substitute (quantitative structure property relationship (QSPR) model and Caco-2 permeability in vitro-in vivo correlation model) and midazolam calibration model with Caco-2 scaling were assessed for determining the transplacental clearance (CLPD) of vancomycin. The m-f PBPK model was developed stepwise using Simcyp, incorporating the determined CLPD values as a crucial input parameter for transplacental kinetics.
Results
The developed PBPK model of vancomycin for non-pregnant adults demonstrated excellent predictive performance. By incorporating the CLPD parameterization derived from ex vivo human placenta perfusion experiments, the extrapolated m-f PBPK model consistently predicted maternal and fetal concentrations of vancomycin across diverse doses and distinct gestational ages. However, when the CLPD parameter was derived from alternative prediction methods, none of the extrapolated maternal–fetal PBPK models produced fetal predictions in line with the observed data.
Conclusion
Our study showcased that combination of ex vivo human placenta perfusion experiments and m-f PBPK model has the capability to predict fetal exposure for the large molecular weight drug vancomycin, whereas other in silico approaches failed to achieve the same level of accuracy.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Wesley BD, Sewell CA, Chang CY, Hatfield KP, Nguyen CP. Prescription medications for use in pregnancy-perspective from the US Food and Drug Administration. Am J Obstet Gynecol. 2021;225(1):21–32.
Ren Z, Bremer AA, Pawlyk AC. Drug development research in pregnant and lactating women. Am J Obstet Gynecol. 2021;225(1):33–42.
Bouazza N, Foissac F, Hirt D, Urien S, Benaboud S, Lui G, Treluyer JM. Methodological approaches to evaluate fetal drug exposure. Curr Pharm Des. 2019;25(5):496–504.
Huang SM, Rowland M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clin Pharmacol Ther. 2012;91(3):542–9.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75.
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51(6):365–96.
Freriksen JJM, Schalkwijk S, Colbers AP, Abduljalil K, Russel FGM, Burger DM, Greupink R. Assessment of maternal and fetal dolutegravir exposure by integrating ex vivo placental perfusion data and physiologically-based pharmacokinetic modeling. Clin Pharmacol Ther. 2020;107(6):1352–61.
De Sousa MM, Lui G, Zheng Y, Pressiat C, Hirt D, Valade E, Bouazza N, Foissac F, Blanche S, Treluyer JM, Urien S, Benaboud S. A physiologically-based pharmacokinetic model to predict human fetal exposure for a drug metabolized by several CYP450 pathways. Clin Pharmacokinet. 2017;56(5):537–50.
Zhang Z, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model II: verification of the model for passive placental permeability drugs. Drug Metab Dispos. 2017;45(8):939–46.
De Sousa MM, Hirt D, Vinot C, Valade E, Lui G, Pressiat C, Bouazza N, Foissac F, Blanche S, Lê MP, Peytavin G, Treluyer JM, Urien S, Benaboud S. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81(4):646–57.
Schalkwijk S, Buaben AO, Freriksen JJM, Colbers AP, Burger DM, Greupink R, Russel FGM. Prediction of fetal darunavir exposure by integrating human ex-vivo placental transfer and physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2018;57(6):705–16.
Liu XI, Momper JD, Rakhmanina N, van den Anker JN, Green DJ, Burckart GJ, Best BM, Mirochnick M, Capparelli EV, Dallmann A. Physiologically based pharmacokinetic models to predict maternal pharmacokinetics and fetal exposure to emtricitabine and acyclovir. J Clin Pharmacol. 2020;60(2):240–55.
Abduljalil K, Pansari A, Ning J, Jamei M. Prediction of maternal and fetal acyclovir, emtricitabine, lamivudine, and metformin concentrations during pregnancy using a physiologically based pharmacokinetic modeling approach. Clin Pharmacokinet. 2022;61(5):725–48.
Abduljalil K, Ning J, Pansari A, Pan X, Jamei M. Prediction of maternal and fetoplacental concentrations of cefazolin, cefuroxime, and amoxicillin during pregnancy using bottom-up physiologically based pharmacokinetic models. Drug Metab Dispos. 2022;50(4):386–400.
Stogios PJ, Savchenko A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020;29(3):654–69.
Emoto C, Johnson TN, McPhail BT, Vinks AA, Fukuda T. Using a vancomycin PBPK model in special populations to elucidate case-based clinical PK observations. CPT Pharmacometrics Syst Pharmacol. 2018;7(4):237–50.
Blouin RA, Bauer LA, Miller DD. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575–80.
Boeckh M, Lode H, Borner K, Höffken G, Wagner J, Koeppe P. Pharmacokinetics and serum bactericidal activity of vancomycin alone and in combination with ceftazidime in healthy volunteers. Antimicrob Agents Chemother. 1988;32(1):92–5.
Healy DP, Polk RE, Garson ML, Rock DT, Comstock TJ. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob Agents Chemother. 1987;31(3):393–7.
Krogstad DJ, Moellering RC Jr, Greenblatt DJ. Single-dose kinetics of intravenous vancomycin. J Clin Pharmacol. 1980;20(4):197–201.
Cutler NR, Narang PK, Lesko LJ, Ninos M, Power M. Vancomycin disposition: the importance of age. Clin Pharmacol Ther. 1984;36(6):803–10.
Healy DP, Sahai JV, Fuller SH, Polk RE. Vancomycin-induced histamine release and “red man syndrome”: comparison of 1- and 2-hour infusions. Antimicrob Agents Chemother. 1990;34(4):550–4.
Lodise TP, Drusano GL, Butterfield JM, Scoville J, Gotfried M, Rodvold KA. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob Agents Chemother. 2011;55(12):5507–11.
Towers CV, Weitz B. Transplacental passage of vancomycin. J Matern Fetal Neonatal Med. 2018;31(8):1021–4.
Reyes MP, Ostrea EM Jr, Cabinian AE, Schmitt C, Rintelmann W. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161(4):977–81.
Laiprasert J, Klein K, Mueller BA, Pearlman MD. Transplacental passage of vancomycin in noninfected term pregnant women. Obstet Gynecol. 2007;109(5):1105–10.
Shin WG, Lee MG, Lee MH, Kim ND. Pharmacokinetics of drugs in blood. VII: unusual distribution and blood storage effect of vancomycin. Biopharm Drug Dispos. 1992;13(4):305–10.
US FDA. Vancomycin Injection label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050671s024lbl.pdf. Acessed on January 10, 2024.
Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51(1):1–13.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Nanovskaya T, Patrikeeva S, Zhan Y, Fokina V, Hankins GD, Ahmed MS. Transplacental transfer of vancomycin and telavancin. Am J Obstet Gynecol. 2012;207(4):331.e331-336.
Nanovskaya T, Deshmukh S, Brooks M, Ahmed MS. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;300(1):26–33.
Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007;8(7):676–84.
Hurdle JG, Heathcott AE, Yang L, Yan B, Lee RE. Reutericyclin and related analogues kill stationary phase Clostridium difficile at achievable colonic concentrations. J Antimicrob Chemother. 2011;66(8):1773–6.
Ndayishimiye J, Cao Y, Kumeria T, Blaskovich MAT, Falconer JR, Popat A. Engineering mesoporous silica nanoparticles towards oral delivery of vancomycin. J Mater Chem B. 2021;9(35):7145–66.
De Sousa MM, Hirt D, Urien S, Valade E, Bouazza N, Foissac F, Blanche S, Treluyer JM, Benaboud S. Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women. Br J Clin Pharmacol. 2015;80(5):1031–41.
Mian P, van den Anker JN, van Calsteren K, Annaert P, Tibboel D, Pfister M, Allegaert K, Dallmann A. Physiologically based pharmacokinetic modeling to characterize acetaminophen pharmacokinetics and N-Acetyl-p-Benzoquinone Imine (NAPQI) formation in non-pregnant and pregnant women. Clin Pharmacokinet. 2020;59(1):97–110.
Winiwarter S, Bonham NM, Ax F, Hallberg A, Lennernäs H, Karlén A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J Med Chem. 1998;41(25):4939–49.
Sun D, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D, Fleisher D, Lee KD, Amidon GL. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm Res. 2002;19(10):1400–16.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number: 82104306), the Science and Technology Innovation Program of Hunan Province (grant number: 2022RC1229), and the Fundamental Research Funds for the Central Universities of Central South University (grant number: 2024ZZTS0986). Certara UK Limited (Simcyp Division) granted access to the Simcyp Simulators through a sponsored academic licence (subject to conditions).
Author information
Authors and Affiliations
Contributions
Yunan Yan and Qiushi Wang developed the PBPK model and write the manuscript. Wei Wu and Hanxi Yi reviewed the manuscript. Feifan Xie conceived the study idea, reviewed, and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, Y., Wang, Q., Wu, W. et al. Evaluation of Various Approaches to Estimate Transplacental Clearance of Vancomycin for Predicting Fetal Concentrations using a Maternal–Fetal Physiologically Based Pharmacokinetic Model. Pharm Res (2024). https://doi.org/10.1007/s11095-024-03705-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-024-03705-2