Abstract
Purpose
To revise the IVIVC considering the physiologically sound Finite Absorption Time (F.A.T.) and Finite Dissolution Time (F.D.T.) concepts.
Methods
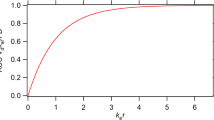
The estimates τ and τd for F.A.T. and F.D.T., respectively are constrained by the inequality τd ≤ τ; their relative magnitude is dependent on drug’s BCS classification. A modified Levy plot, which includes the time estimates for τ and τd was developed. IVIVC were also considered in the light of τ and τd estimates. The modified Levy plot of theophylline, a class I drug, coupled with the rapid (30 min) and very rapid (15 min) dissolution time limits showed that drug dissolution/absorption of Class I drugs takes place in less than an hour. We reanalyzed a carbamazepine (Tegretol) bioequivalence study using PBFTPK models to reveal its complex absorption kinetics with two or three stages.
Results
The modified Levy plot unveiled the short time span (~ 2 h) of the in vitro dissolution data in comparison with the duration of in vivo dissolution/absorption processes (~ 17 h). Similar results were observed with the modified IVIVC plots. Analysis of another set of carbamazepine data, using PBFTPK models, confirmed a three stages absorption process. Analysis of steady-state (Tegretol) data from a paediatric study using PBFTPK models, revealed a single input stage of duration 3.3 h. The corresponding modified Levy and IVIVC plots were found to be nonlinear.
Conclusions
The consideration of Levy plots and IVIVC in the light of the F.A.T. and F.D.T. concepts allows a better physiological insight of the in vitro and in vivo drug dissolution/absorption processes.













Similar content being viewed by others
Abbreviations
- F.A.T.:
-
Finite Absorption Time
- F.D.T.:
-
Finite Dissolution Time
- IVIVC:
-
In vitro in vivo correlations
- PBPK:
-
Physiologically Based Pharmacokinetic (PBPK) models
- PBFTPK:
-
Physiologically Based Finite Time Pharmacokinetic (PBFTPK) models
References
Macheras P. On an unphysical hypothesis of Bateman equation and its implications for pharmacokinetics. Pharm Res. 2019;36:94. https://doi.org/10.1007/s11095-019-2633-4.
Tsekouras AA, Macheras P. Columbus’ egg: Oral drugs are absorbed in finite time. Eur J Pharm Sci. 2022;176:106265. https://doi.org/10.1016/j.ejps.2022.106265.
Macheras P, Tsekouras AA. The Finite Absorption Time (FAT) concept en route to PBPK modeling and pharmacometrics. J Pharmacokinet Pharmacodyn. 2023;50:5–10. https://doi.org/10.1007/s10928-022-09832-w.
Macheras P, Tsekouras AA. Revising Oral Pharmacokinetics, Bioavailability and Bioequivalence Based on the Finite Absorption Time Concept. Cham: Springer; 2023. https://doi.org/10.1007/978-3-031-20025-0.
Macheras P, Chryssafidis P. Revising pharmacokinetics of oral drug absorption: I models based on biopharmaceutical/physiological and finite absorption time concepts. Pharm Res. 2020;37:187. https://doi.org/10.1007/s11095-020-02894-w. Erratum. Pharm Res 2020;37:206. https://doi.org/10.1007/s11095-020-02935-4.
Chryssafidis P, Tsekouras AA, Macheras P. Re-writing oral pharmacokinetics using physiologically based finite time pharmacokinetic (PBFTPK) models. Pharm Res. 2022;39:691–701. https://doi.org/10.1007/s11095-022-03230-0.
Wu D, Tsekouras AA, Macheras P, Kesisoglou F. Physiologically based pharmacokinetic models under the prism of the finite absorption time concept. Pharm Res. 2023;40:419–29. https://doi.org/10.1007/s11095-022-03357-0.
Alimpertis N, Tsekouras AA, Macheras P. Revamping Biopharmaceutics-Pharmacokinetics with Scientific and Regulatory Implications for Oral Drug Absorption. Pharm Res. 2023;40:2167–75. https://doi.org/10.1007/s11095-023-03578-x.
United States Pharmacopeia (2022). General Chapter, 〈1088〉 in Vitro and in Vivo Evaluation of Oral Dosage Forms. USP-NF. Rockville, MD: United States Pharmacopeia. https://doi.org/10.31003/USPNF_M99807_04_0.
FDA Guidance for Industry Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. Office of Training and Communications Division of Communications Management. The Drug Information Branch, HFD-210 5600 Fishers Lane Rockville, MD 20857.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. https://doi.org/10.1023/a:1016212804288.
Wu C, Benet L. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. https://doi.org/10.1007/s11095-004-9004-4.
Charalabidis A, Sfouni M, Bergström C, Macheras P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond guidelines. Int J Pharm. 2019;566:264–81. https://doi.org/10.1016/j.ijpharm.2019.05.041.
Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Guidance for Industry U.S. Department of Health and Human Services. 2017. http://resource.nlm.nih.gov/101720038
European Medicines Agency. Committee for medicinal products for human use (CHMP) Guideline on the investigation of bioequivalence. London, Jan. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf
Suarez-Sharp S, Li M, Duan J, Shah H, Seo P. Regulatory Experience with In Vivo In Vitro Correlations (IVIVC) in New Drug Applications. AAPS J. 2016;18(6):1379–90. https://doi.org/10.1208/s12248-016-9966-2.
Mircioiu C, Mircioiu I, Voicu V, Miron D. Dissolution–bioequivalence non-correlations. Basic Clin Pharmacol Toxicol. 2005;96:262–4. https://doi.org/10.1111/j.1742-7843.2005.pto960324.x.
Dokoumetzidis A, Macheras P. IVIVC of controlled release formulations: Physiological-dynamical reasons for their failure. J Control Release. 2008;129:76–8. https://doi.org/10.1016/j.jconrel.2008.04.005.
Certrara, Levy-Plots, https://onlinehelp.certara.com/phoenix/8.3/topics/levyplots.htm. Accessed 4 Jan 2024
Chryssafdis P, Tsekouras AA, Macheras P. Revising pharmacokinetics of oral drug absorption: II bioavailability bioequivalence considerations. Pharm Res. 2021;38:1345–56. https://doi.org/10.1007/s11095-021-03078-w.
Saelim N, Suksawaeng K, Chupan J, Techatanawat I. Biopharmaceutics classification system (BCS)-based biowaiver for immediate release solid oral dosage forms of moxifloxacin hydrochloride (Moxiflox GPO) manufactured by the Government Pharmaceutical Organization (GPO). Asian J Pharmaceut Sci. 2016;11:235–6. https://doi.org/10.1016/j.ajps.2015.11.016.
Lange ADC, Batistel AP, Sfair LL, Carlosso J, Volpato NM, Schapoval ESS. Sitagliptin Phosphate: Development of a Dissolution Method for Coated Tablets Based on In Vivo Data for Improving Medium Sensitivity. Dissolution Technol. 2014;2014(5):17–22. https://doi.org/10.14227/DT210214P17.
Ochekpe NA, Ngwuluka NC, Owolayo H, Fashedemi T. Dissolution Profiles of Three Brands of Lamivudine and Zidovudine Combinations in the Nigerian Market. Dissolution Technol. 2006;2006(11):12–7. https://doi.org/10.14227/DT130406P12.
Pleoger GF, Quizon PM, Abrahamsson B, Cristofoletti R, Groot DW, Parr A, Langguth P, Polli JE, Shah VP, Tajiri T, Mehta MU, Dressman J. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Cephalexin Monohydrate. J Pharm Sci. 2020;109:1846–62. https://doi.org/10.1016/j.xphs.2020.03.025.
Prakash K, Raju NP, Kumari SK, Narasu LM. Solubility and Dissolution Rate Determination of Different Antiretroviral Drugs in Different pH Media Using UV Visible Spectrophotometer. E-J Chem. 2008;5(S2):1159–64. https://doi.org/10.1155/2008/125917.
Nair A, Abrahamsson B, Barends DM, Groot DW, Kopp S, Polli EJ, Shah VP, Dressman JB. Biowaiver Monographs for Immediate-Release Solid Oral Dosage Forms: Primaquine Phosphate. J Pharm Sci. 2011;101:936–45. https://doi.org/10.1002/jps.23006.
Verbeeck RK, Kanfer I, Löbenberg R, Abrahamsson B, Cristofoletti R, Groot DW, Langguth P, Polli JE, Parr A, Shah VP, Mehta M, Dressman JB. Biowaiver monographs for Immediate Release solid oral dosage forms: Enalapril. J Pharm Sci. 2017;106:P1933-1943. https://doi.org/10.1016/j.xphs.2017.04.019.
Plöger GF, Abrahamsson B, Cristofoletti R, Groot DW, Langguth P, Mehta MU, Parr A, Polli JE, Shah VP, Tajiri T, Dressman JB. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Proguanil Hydrochloride. J Pharm Sci. 2018;107:1761–72. https://doi.org/10.1016/j.xphs.2018.03.009.
Akdag Y, Gulsun T, Izat N, Oner L, Sahin S. Comparison of Dissolution Profiles and Apparent Permeabilities of Commercially Available Metformin Hydrochloride Tablets in Turkey. Dissolution Technol. 2020;2020(2):22–9. https://doi.org/10.14227/DT270120P22.
Susantakumar P, Gaur A, Sharma P. Feasibility Biowaiver Extension of Immediate Release Oral Acyclovir 800 mg Tablet Formulations: A BCS Class III Drug. Int J Pharm Pharmaceut Sci. 2011;3;384–391. https://innovareacademics.in/journal/ijpps/Vol3Issue4/2689.pdf . Accessed 4 Jan 2024
Jantratid E, Prakongpan S, Amidon GL, Dressman JB. Feasibility of Biowaiver Extension to Biopharmaceutics Classification System Class III Drug Products: Cimetidine. Clin Pharmacokinet. 2006;45:385–99. https://doi.org/10.2165/00003088-200645040-00004.
Hassouna MEM, Issa YM, Zayed AG. A comparative study of the in-vitro dissolution profiles of paracetamol and caffeine combination in different formulations using HPLC. J Applied Pharm Sci. 2012;2:52–9. https://doi.org/10.7324/JAPS.2012.2531.
Kassaye L, Genete G. Evaluation and comparison of in-vitro dissolution profiles for different brands of amoxicillin capsules. Afr Health Sci. 2013;13:369–75. https://doi.org/10.4314/ahs.v13i2.25.
Thambavita D, Jayathilake CM, Sandamali KD, Galappatthy P, Jayakody RL. In Vitro Dissolution Testing to Assess Pharmaceutical Equivalence of Selected Amoxicillin Products Available in Sri Lanka: A Post-Marketing Study. Dissolution Technol. 2019;2019(2):56–61. https://doi.org/10.14227/DT260119P56.
Strauch S, Jantratid T, Dressman JB, Junginger UE, Kopp S, Midha KK, Shah VP, Stavchansky S, Barends DM. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Lamivudine. J Pharm Sci. 2011;100:2054–63. https://doi.org/10.1002/jps.22449.
FDA, Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances. Guidance for Industry, 2018. https://www.fda.gov/media/92988/download. Accessed 4 Jan 2024
González-García I, Mangas-Sanjuan V, Merino-Sanjuán M, Álvarez-Álvarez C, Díaz-Garzón Marco J, Rodríguez-Bonnín MA, Langguth T, Torrado-Durán JJ, Langguth P, García-Arieta A, Bermejo M. IVIVC approach based on carbamazepine bioequivalence studies combination. Pharmazie. 2017;72:449–57. https://doi.org/10.1691/ph.2017.7011.
Sjogren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernas H, Abrahamsson B, Tannergren C. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: Application of the mechanistic absorption model GI-Sim. Eur J Pharm Sci. 2013;49:679–98. https://doi.org/10.1016/j.ejps.2013.05.019.
Hartley R, Aleksandrowicz J, Bowmer CJ, Cawood A, Forsythe WI. Dissolution and relative bioavailability of two carbamazepine preparations for children with epilepsy. J Pharm Pharmacol. 1991;43:117–9. https://doi.org/10.1111/j.2042-7158.1991.tb06644.x.
Pawar G, Wu F, Zhao L, Fang L, Burckart GJ, Feng K, Mousa YM, Al Shoyaib A, Jones M-C, Batchelor HK. Integration of Biorelevant Pediatric Dissolution Methodology into PBPK Modeling to Predict. AAPS J. 2023;25:67. https://doi.org/10.1208/s12248-023-00826-1.
Macheras P, Argyrakis P. Gastrointestinal drug absorption: is it time to consider heterogeneity as well as homogeneity? Pharm Res. 1997;14(7):842–7. https://doi.org/10.1023/a:1012183313218.
Charkoftaki G, Kytariolos J, Macheras P. Novel milk-based oral formulations: Proof of concept. Int J Pharm. 2010;390:150–9. https://doi.org/10.1016/j.ijpharm.2010.001.038.
Trabelsi F, Gharavi N, Kalovidouris M, Nikolaidou M. Steady-state bioequivalence 2-way crossover study of two quetiapine prolonged-release 400 mg tablet formulations in normal male and female healthy subjects under fasting conditions. Int J Clin Pharmacol Ther. 2016;54(9):732–42. https://doi.org/10.5414/CP202609.
Macheras P, Koupparis M, Tsaprounis C. Drug dissolution studies in milk using the automated flow-injection serial dynamic dialysis technique. Int J Pharm. 1986;33:125–36. https://doi.org/10.1016/0378-5173(86)90046-3.
Macheras P, Koupparis M, Apostolelli E. Dissolution of 4 controlled-release theophylline formulations in milk. Int J Pharm. 1987;36:73–9. https://doi.org/10.1016/0378-5173(87)90239-0.
Jede C, Henze LJ, Meiners K, Bogdahn M, Wedel M, van Axel V. Development and Application of a Dissolution-Transfer-Partitioning System (DTPS) for Biopharmaceutical Drug Characterization. Pharmaceutics. 2023;15(4):1069. https://doi.org/10.3390/pharmaceutics15041069.
Phillips DJ, Pygall SR, Cooper VB, Mann JC. Overcoming sink limitations in dissolution testing: a review of traditional methods and the potential utility of biphasic systems. J Pharm Pharmacol. 2012;64(11):1549–59. https://doi.org/10.1111/j.2042-7158.2012.01523.x.
Acknowledgements
This work is dedicated to all students who maintain respect to their teachers.
Funding
This work received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
We list below the analytical expressions for 4 cases of drug concentration in the blood as a function of time after oral administration for the one-compartment model with zero-order, finite time absorption kinetics in one or more stages.
-
1
Single input stage of duration τ.
For \(0<t\le \tau\),
For \(\tau <t\),
-
2
Two consecutive input stages of duration τ1 and τ2.
For \(0<t\le {\tau }_{1}\),
For \({\tau }_{1}<t\le {{\tau }_{1}+\tau }_{2}\),
For \({\tau }_{1}+{\tau }_{2}<t\),
-
3
n consecutive input stages each of duration τi.
For \(0<t\le {\tau }_{1}\),
For \(\sum_{j=1}^{i-1}{\tau }_{j}<t\le \sum_{j=1}^{i}{\tau }_{j}\),
For \(\sum_{j=1}^{n}{\tau }_{j}<t\),
-
4
One input stage of duration τ coupled with n identical doses administered at equal Δt intervals.
For \(i\Delta t<t\le \tau +i\Delta t\), where i is an integer with \(0\le i<n\)
where \(C\left(0\right)=0\)
For \(\tau +i\Delta t<t\le \left(i+1\right)\Delta t\) or \(\tau +n\Delta t<t\),
The latter set of equations was used to generate Fig. 10 and analyze the data shown in Fig. 11.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alimpertis, N., Simitopoulos, A., Tsekouras, A.A. et al. IVIVC Revised. Pharm Res 41, 235–246 (2024). https://doi.org/10.1007/s11095-024-03653-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03653-x