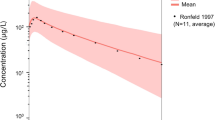
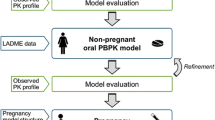
Abstract
Objective
This study aims to develop physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) predictive models for nifedipine in pregnant women, enhancing precision medicine and reducing adverse reactions for both mothers and infants.
Methods
A PBPK/PD model was constructed using PK-Sim, MoBi, and MATLAB software, integrating literature and pregnancy-specific physiological information. The process involved: (1) establishing and validating a PBPK model for serum clearance after intravenous administration in non-pregnant individuals, (2) establishing and validating a PBPK model for serum clearance after oral administration in non-pregnant individuals, (3) constructing and validating a PBPK model for enzyme clearance after oral administration in non-pregnant individuals, and (4) adjusting the PBPK model structure and enzyme parameters according to pregnant women and validating it in oral administration. (5) PK/PD model was explored through MATLAB, and the PBPK and PK/PD models were integrated to form the PBPK/PD model.
Results
The Nifedipine PBPK model's predictive accuracy was confirmed by non-pregnant and pregnant validation studies. The developed PBPK/PD model accurately predicted maximum antihypertensive effects for clinical doses of 5, 10, and 20 mg. The model suggested peak effect at 0.86 h post-administration, achieving blood pressure reductions of 5.4 mmHg, 14.3 mmHg, and 21.3 mmHg, respectively. This model provides guidance for tailored dosing in pregnancy-induced hypertension based on targeted blood pressure reduction.
Conclusion
Based on available literature data, the PBPK/PD model of Nifedipine in pregnancy demonstrated good predictive performance. It will help optimize individualized dosing of Nifedipine, improve treatment outcomes, and minimize the risk of adverse reactions in mothers and infants.









Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are derived from publicly available databases and previously published literature. All data sources are appropriately cited within the manuscript. Additional information regarding data availability can be obtained from the corresponding author upon reasonable request.
References
Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. 2021;21(1):364.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Compendium on the pathophysiology and treatment of hypertension preeclampsia. Circ Res. 2019;124(7):1094–1112.
Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8.
Hennington BS, Alexander BT. Linking intrauterine growth restriction and blood pressure: insight into the human origins of cardiovascular disease. Circulation. 2013;128(20):2179–80.
Cohen E, Wong FY, Horne RSC, Yiallourou SR. Intrauterine growth restriction: impact on cardiovascular development and function throughout infancy. Pediatr Res. 2016;79(6):821–30.
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–241.
Sheffield JS, Siegel D, Mirochnick M, Heine RP, Nguyen C, Bergman KL, et al. Designing Drug Trials: Considerations for Pregnant Women. Clin Infect Dis. 2014;59(suppl_7):S437–44.
Pinheiro EA, Stika CS. Drugs in pregnancy: pharmacologic and physiologic changes that affect clinical care. Semin Perinatol. 2020;44(3): 151221.
Weld ED, Bailey TC, Waitt C. Ethical issues in therapeutic use and research in pregnant and breastfeeding women. Brit J Clinical Pharma. 2022;88(1):7–21.
McKiever M, Frey H, Costantine MM. Challenges in conducting clinical research studies in pregnant women. J Pharmacokinet Pharmacodyn. 2020;47(4):287–93.
Tan YM, Chan M, Chukwudebe A, Domoradzki J, Fisher J, Hack CE, et al. PBPK model reporting template for chemical risk assessment applications. Regul Toxicol Pharmacol. 2020;115: 104691.
Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B. 2016;6(5):430–40.
Ke A, Nallani S, Zhao P, Rostami-Hodjegan A, Unadkat J. A PBPK model to predict disposition of CYP3A-metabolized drugs in pregnant women: verification and discerning the site of CYP3A induction. CPT: Pharmacometrics & Systems Pharmacology. 2012;1(9):3.
Abduljalil K, Pansari A, Jamei M. Prediction of maternal pharmacokinetics using physiologically based pharmacokinetic models: assessing the impact of the longitudinal changes in the activity of CYP1A2, CYP2D6 and CYP3A4 enzymes during pregnancy. J Pharmacokinet Pharmacodyn. 2020;47(4):361–83.
Dallmann A, Ince I, Coboeken K, Eissing T, Hempel G. A physiologically based pharmacokinetic model for pregnant women to predict the pharmacokinetics of drugs metabolized via several enzymatic pathways. Clin Pharmacokinet. 2018;57(6):749–68.
Zou H, Banerjee P, Leung SSY, Yan X. Application of pharmacokinetic-pharmacodynamic modeling in drug delivery: development and challenges. Front Pharmacol. 2020;3(11):997.
Tuntland T, Ethell B, Kosaka T, Blasco F, Zang RX, Jain M, et al. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front Pharmacol [Internet]. 2014 Jul 28 [cited 2023 Jul 30];5. Available from: https://doi.org/10.3389/fphar.2014.00174/abstract.
Bloomingdale P, Bumbaca-Yadav D, Sugam J, Grauer S, Smith B, Antonenko S, et al. PBPK-PD modeling for the preclinical development and clinical translation of tau antibodies for Alzheimer’s disease. Front Pharmacol. 2022;2(13): 867457.
Darakjian LI, Kaddoumi A. Physiologically based pharmacokinetic/pharmacodynamic model for caffeine disposition in pregnancy. Mol Pharmaceutics. 2019;16(3):1340–9.
Wen HN, He QF, Xiang XQ, Jiao Z, Yu JG. Predicting drug-drug interactions with physiologically based pharmacokinetic/pharmacodynamic modelling and optimal dosing of apixaban and rivaroxaban with dronedarone co-administration. Thromb Res. 2022;218:24–34.
Gajendran J, Krämer J, Shah VP, Langguth P, Polli J, Mehta M, et al. Biowaiver monographs for immediate-release solid oral dosage forms: nifedipine. J Pharm Sci. 2015;104(10):3289–98.
Otto J, Lesko LJ. Protein binding of nifedipine. J Pharm Pharmacol. 2011;38(5):399–400.
Clarysse S, Psachoulias D, Brouwers J, Tack J, Annaert P, Duchateau G, et al. Postprandial changes in solubilizing capacity of human intestinal fluids for BCS Class II drugs. Pharm Res. 2009;26(6):1456–66.
Al Ameri MN, Nayuni N, Anil Kumar KG, Perrett D, Tucker A, Johnston A. The differences between the branded and generic medicines using solid dosage forms: In-vitro dissolution testing. Results Pharma Sci. 2012;2:1–8.
Renwick AG, Le Vie J, Challenor VF, Waller DG, Gruchy B, George CF. Factors affecting the pharmacokinetics of nifedipine. Eur J Clin Pharmacol. 1987;32(4):351–5.
Patki KC, von Moltke LL, Greenblatt DJ. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes P450: role of CYP3A4 and CYP3a5. Drug Metab Dispos. 2003;31(7):938–44.
Rashid T, Martin U, Clarke H, Waller D, Renwick A, George C. Factors affecting the absolute bioavailability of nifedipine. Br J Clin Pharmacol. 1995;40(1):51–8.
Anderson GD, Carr DB. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs: Clin Pharmacokinet. 2009;48(3):159–68.
Logoyda L. HPLC-MS/MS method development for the quantitative determination of nifedipine for Caco-2 permeability assay. PHAR. 2020;67(2):83–8.
Ahsan C, Renwick A, Macklin B, Challenor V, Waller D, George C. Ethnic differences in the pharmacokinetics of oral nifedipine. Br J Clin Pharmacol. 1991;31(4):399–403.
Raemsch, K D, & Sommer, J (1983). Pharmacokinetics and metabolism of nifedipine. Hypertension, 5(4_pt_2), II18.
Foster TS, Hamann SR, Richards VR, Bryant PJ, Graves DA, McALLISTER RG. Nifedipine kinetics and bioavailability after single intravenous and oral doses in normal subjects. J Clin Pharmacol. 1983;23(4):161–70.
Harris RZ, Inglis AML, Miller AK, Thompson KA, Finnerty D, Patterson S, et al. Rosiglitazone has no clinically significant effect on nifedipine pharmacokinetics. J Clin Pharmacol. 1999;39(11):1189–94.
Bauman J. The role of pharmacokinetics, drug interactions and pharmacogenetics in the acquired long QT syndrome. Eur Heart J Suppl. 2001;3:K93-100.
Iacopetta D, Ceramella J, Catalano A, Scali E, Scumaci D, Pellegrino M, et al. Impact of cytochrome P450 enzymes on the phase I metabolism of drugs. Appl Sci. 2023;13(10):6045.
Dallmann A. Applied concepts in PBPK modeling how to extend an open systems pharmacology model to the special population of pregnant women. CPT Pharmacometrics Syst Pharmacol. 2018;7(7):419–31.
Barton JR, Prevost RR, Wilson DA, Whybrew WD, Sibai BM. Nifedipine pharmacokinetics and pharmacodynamics during the immediate postpartum period in patients with preeclampsia. Am J Obstet Gynecol. 1991;165(4):951–4.
Prevost RR. Oral Nifedipine Pharmacokinetics in Pregnancy‐Induced Hypertension.
Walters BNJ, Redman CWG. Treatment of severe pregnancy-associated hypertension with the calcium antagonist nifedipine. BJOG: An International Journal of O&G. 1984;91(4):330–6.
Hypertension in pregnancy: Number 219 - January 1996 (replaces no. 91, February 1986). Int J Gynecol Obstet. 1996;53(2):175–83.
Committee on Practice Bulletins—Obstetrics. Gestational Hypertension and Preeclampsia. Replaces Practice Bulletin No. 202, December 2018.
Chinese Medical Association Obstetrics and Gynecology Branch Pregnancy-Induced Hypertension Disease Study Group. Guidelines for the Diagnosis and Treatment of Pregnancy-Induced Hypertension Diseases 2020. Chin J Obstet Gynecol, 2020;55(4)
Funding
This research was supported by grants from the Shenzhen-Hong Kong-Macau Science and Technology Program (Category C) of Shenzhen Science and Technology Innovation Commission (SGDX20210823103802016), Industry-University-Research Cooperation Project and Zhuhai-Hong Kong-Macao Cooperation Project from Zhuhai Science and Technology Innovation Bureau (ZH22017002210010PWC) and the General Program of Guangdong Province Natural Science Foundation under Grant No. 2022A1515012405.
Author information
Authors and Affiliations
Contributions
Xinyang Liu, Wei Wang, Jingsi Chen, Dunjin Chen, Yong Tao and Defang Ouyang contributed to the conception and design of the study. Xinyang Liu, Wei Wang and Defang Ouyang were involved in data collection, analysis, and interpretation. Xinyang Liu contributed to drafting the manuscript. Wei Wang and Defang Ouyang provided critical revisions. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest regarding this research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Wang, W., Chen, J. et al. PBPK/PD Modeling of Nifedipine for Precision Medicine in Pregnant Women: Enhancing Clinical Decision-Making for Optimal Drug Therapy. Pharm Res 41, 63–75 (2024). https://doi.org/10.1007/s11095-023-03638-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03638-2