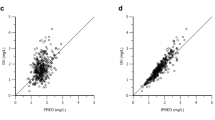
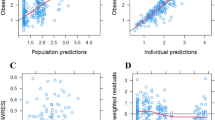
Abstract
Aims
Dasatinib, a second-generation tyrosine kinase inhibitor of BCR-ABL 1, used for first-line treatment of Philadelphia chromosome-positive chronic myeloid leukemia (CML), exhibits high pharmacokinetic (PK) variability. However, its PK data in Chinese patients with CML remains rarely reported to date. Thus, we developed a population pharmacokinetic (PPK) model of dasatinib in Chinese patients and identified the covariate that could explain the individual variability of PK for optimal individual administration.
Methods
PPK modeling for dasatinib was performed based on 754 plasma concentrations obtained from 140 CML patients and analysis of various genetic and physicochemical parameters. Modeling was performed with nonlinear mixed-effects (NLME) using Phoenix NLME. The finally developed model was evaluated using internal and external validation. Monte Carlo simulations were used to predict drug exposures at a steady state for various dosages.
Results
The PK of dasatinib were well described by a two-compartment with a log-additive residual error model. Patients in the current study had a relatively low estimate of CL/F (126 L/h). A significant association was found between the covariate of age and CL/F of dasatinib, which was incorporated into the final model. None of the genetic factors was confirmed as a significant covariate for dasatinib. The results of external validation with 140 samples from 36 patients were acceptable. Simulation results showed significantly higher exposures in elderly patients.
Conclusions
This study’s findings suggested that low-dose dasatinib would be better suited for Chinese patients, and the dosage can be appropriately reduced according to the increase of age, especially for the elderly.



Similar content being viewed by others
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
References
Apperley JF. Chronic myeloid leukaemia. Lancet. 2015;385(9976):1447–59. https://doi.org/10.1016/s0140-6736(13)62120-0.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–84. https://doi.org/10.1038/s41375-020-0776-2.
He S, Bian J, Shao Q, Zhang Y, Hao X, Luo X, et al. Therapeutic Drug Monitoring and Individualized Medicine of Dasatinib: Focus on Clinical Pharmacokinetics and Pharmacodynamics. Front Pharmacol. 2021;12:797881. https://doi.org/10.3389/fphar.2021.797881.
Takahashi N, Miura M, Scott SA, Niioka T, Sawada K. Pharmacokinetics of dasatinib for Philadelphia-positive acute lymphocytic leukemia with acquired T315I mutation. J Hematol Oncol. 2012;5:23. https://doi.org/10.1186/1756-8722-5-23.
Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen TT, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a Phase III study. Clin Pharmacol. 2013;5:85–97. https://doi.org/10.2147/CPAA.S42796.
Mizuta S, Sawa M, Tsurumi H, Matsumoto K, Miyao K, Hara T, et al. Plasma concentrations of dasatinib have a clinical impact on the frequency of dasatinib dose reduction and interruption in chronic myeloid leukemia: an analysis of the DARIA 01 study. Int J Clin Oncol. 2018;23(5):980–8. https://doi.org/10.1007/s10147-018-1300-9.
Rousselot P, Mollica L, Guilhot J, Guerci A, Nicolini FE, Etienne G, et al. Dasatinib dose optimisation based on therapeutic drug monitoring reduces pleural effusion rates in chronic myeloid leukaemia patients. Brit J Haematol. 2021;194(2):393–402. https://doi.org/10.1111/bjh.17654.
Dai G, Pfister M, Blackwood-Chirchir A, Roy A. Importance of characterizing determinants of variability in exposure: application to dasatinib in subjects with chronic myeloid leukemia. J Clin Pharmacol. 2008;48(11):1254–69. https://doi.org/10.1177/0091270008320604.
Yoshitsugu H, Imai Y, Seriu T, et al. Markov Chain Monte Carlo Bayesian Analysis for Population Pharmacokinetics of Dasatinib in Japanese Adult Subjects with Chronic Myeloid Leukemia and Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. 臨床薬理:Japanese Journal of Clinical. 2012;43(1):29–41.
Bloomfield C, Staatz CE, Unwin S, Hennig S. Assessing Predictive Performance of Published Population Pharmacokinetic Models of Intravenous Tobramycin in Pediatric Patients. Antimicrob Agents Chemother. 2016;60(6):3407–14. https://doi.org/10.1128/AAC.02654-15.
Hara M, Masui K, Eleveld DJ, Struys MMRF, Uchida O. Predictive performance of eleven pharmacokinetic models for propofol infusion in children for long-duration anaesthesia. Brit J Anaesth. 2017;118(3):415–23. https://doi.org/10.1093/bja/aex007.
Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305–25. https://doi.org/10.1007/s40262-014-0137-2.
Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol Pharm Bull. 2015;38(5):645–54.
Hao-ming S, Ting-ting Z, Min S, Xiao-juan M, Wei-wei Z, Lin Y, et al. Pharmacokinetics of dasatinib tablets in Chinese healthy volunteers. Chin J New Drugs. 2013;22(17):5.
Jun K, Nan C, Haixia F, Taijun H, Min S, Hao J. Pharmacokinefics of generic dasatinib in the management of chronic myeloid leukemia in the choronie phase. Chin J Hematol. 2016;37(11):4.
Tao C. Clinical Study on Domestic-Made Dasatinib Tablets for Treating Patients with Chronic Myeloid Leukemia in Chronic Phase and Its Pharmacokinetics. China Pharmaceuticals. 2018;27(11):3.
Kim D-W, Goh Y-T, Hsiao H-H, Caguioa PB, Kim D, Kim W-S, et al. Clinical profile of dasatinib in Asian and non-Asian patients with chronic myeloid leukemia. Int J Hematol. 2009;89(5):664–72. https://doi.org/10.1007/s12185-009-0326-1.
Yang F, Zhang L, Zhao BB, Zhang JL, Liu XT, Li X, et al. Population Pharmacokinetics and Safety of Dasatinib in Chinese Children with Core-Binding Factor Acute Myeloid Leukemia. Clin Pharmacokinetic. 2022;61(1):71–81. https://doi.org/10.1007/s40262-021-01054-6.
de Wildt SN, Tibboel D, Leeder JS. Drug metabolism for the paediatrician. Arch Dis Child. 2014;99(12):1137–42. https://doi.org/10.1136/archdischild-2013-305212.
Itamura H, Kubota Y, Shindo T, Ando T, Kojima K, Kimura S. Elderly Patients With Chronic Myeloid Leukemia Benefit From a Dasatinib Dose as Low as 20 mg. Clin Lymphoma Myeloma Leuk. 2017;17(6):370–4. https://doi.org/10.1016/j.clml.2017.02.023.
Murai K, Ureshino H, Kumagai T, Tanaka H, Nishiwaki K, Wakita S, et al. Low-dose dasatinib in older patients with chronic myeloid leukaemia in chronic phase (DAVLEC): a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2021;8(12):e902–11. https://doi.org/10.1016/s2352-3026(21)00333-1.
Iriyama N, Ohashi K, Hashino S, Kimura S, Nakaseko C, Takano H, et al. The Efficacy of Reduced-dose Dasatinib as a Subsequent Therapy in Patients with Chronic Myeloid Leukemia in the Chronic Phase: The LD-CML Study of the Kanto CML Study Group. Intern Med. 2018;57(1):17–23. https://doi.org/10.2169/internalmedicine.9035-17.
Maia RC, Vasconcelos FC, Souza PS, Rumjanek VM. Towards Comprehension of the ABCB1/P-Glycoprotein Role in Chronic Myeloid Leukemia. Molecules. 2018;23(1). https://doi.org/10.3390/molecules23010119.
Dessilly G, Panin N, Elens L, Haufroid V, Demoulin JB. Impact of ABCB1 1236C > T-2677G > T-3435C > T polymorphisms on the anti-proliferative activity of imatinib, nilotinib, dasatinib and ponatinib. Sci Rep. 2016;6:29559. https://doi.org/10.1038/srep29559.
Antonia H, Tolson, Hongbing, Wang. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62(13):1238–49.
Gong L, Zhang CM, Lv JF, Zhou HH, Fan L. Polymorphisms in cytochrome P450 oxidoreductase and its effect on drug metabolism and efficacy. Pharmacogenet Genomics. 2017;27(9):1.
Funding
This work was supported by the Beijing Municipal Natural Science Foundation (grant number 7192218).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This study was conducted following the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the People’s Hospital of Peking University (2022PHB095-001).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, S., Zhao, J., Bian, J. et al. Population Pharmacokinetics and Pharmacogenetics Analyses of Dasatinib in Chinese Patients with Chronic Myeloid Leukemia. Pharm Res 40, 2413–2422 (2023). https://doi.org/10.1007/s11095-023-03603-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03603-z