Abstract
Background
Imatinib is presently the first-line choice for the treatment of chronic myeloid leukemia. However, there are limited real-world data on Chinese patients to support individualized medicine. This work aims to characterize population pharmacokinetics in Chinese patients with chronic myeloid leukemia, investigate the effects of several covariates on imatinib exposure, and provide support for personalized medicine and dose reduction.
Methods
A total of 230 patients with chronic myeloid leukemia were enrolled, and 424 steady-state concentration measurements were taken to perform the population pharmacokinetic analysis and Monte Carlo simulations with Phoenix NLME software. The effects of the demographic, biological, and pharmacogenetic (ten SNP corresponding to CYP3A4, CYP3A5, ABCB1, ABCG2, SCL22A1 and POR) covariates on clearance were evaluated.
Results
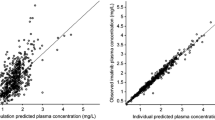
A one-compartmental model best-described imatinib pharmacokinetics. The hemoglobin and the estimated glomerular filtration rate (< 85 mL⋅min−1⋅1.73 m2) were associated with imatinib clearance. The genetic polymorphisms related to pharmacokinetics were not found to have a significant effect on the clearance of imatinib. The final model estimates of parameters are: ka (h−1) = 0.329; Vd/F (L) = 270; CL/F (L⋅h−1) = 7.60.
Conclusions
Key covariates in the study population accounting for variability in imatinib exposure are hemoglobin and the estimated glomerular filtration rate. There is some need for caution when treating patients with moderate-to-severe renal impairment and significant hemoglobin changes.





Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Cohen MH, Williams G, Johnson JR et al (2002) Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res 8(5):935–942
García-Ferrer M, Wojnicz A, Mejía G, Koller D, Abad-Santos F (2019) Utility of therapeutic drug monitoring of imatinib, nilotinib, and dasatinib in chronic myeloid leukemia: a systematic review and meta-analysis. Clin Ther 41(12):22
Hochhaus A, Baccarani M, Silver RT et al (2020) European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 34(4):966–984
Deininger MW, Shah NP, Altman JK et al (2020) Chronic myeloid leukemia, version 22021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18(10):1385–1415
Peng B, Lloyd P, Schran H (2005) Clinical pharmacokinetics of imatinib. Clin Pharmacokinet 44(9):879–894
Clarke WA, Chatelut E, Fotoohi AK et al (2021) Therapeutic drug monitoring in oncology: International association of therapeutic drug monitoring and clinical toxicology consensus guidelines for imatinib therapy. Euro J Cancer. 157:428–440
Picard S, Titier K, Etienne G et al (2007) Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 109(8):3496–3499
Larson RA, Druker BJ, Guilhot F et al (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111(8):4022–4028
Koren-Michowitz M, Volchek Y, Naparstek E et al (2012) Imatinib plasma trough levels in chronic myeloid leukaemia: results of a multicentre study CSTI571AIL11TGLIVEC. Hematol Oncol 30(4):200–205
Marin D, Bazeos A, Mahon F-X et al (2010) Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 28(14):2381–2388
Guilhot F, Hughes TP, Cortes J et al (2012) Plasma exposure of imatinib and its correlation with clinical response in the Tyrosine Kinase Inhibitor Optimization and Selectivity Trial. Haematologica 97(5):731–738
Bonate PL (2005) Recommended reading in population pharmacokinetic pharmacodynamics. Aaps j 7(2):E363–E373
FDA. FDA Guidance for Industry on "Population Pharmacokinetics". 1999;
Corral Alaejos Á, Zarzuelo Castañeda A, Jiménez Cabrera S, Sánchez-Guijo F, Otero MJ, Pérez-Blanco JS (2021) External Evaluation of Population Pharmacokinetic Models of Imatinib in Adults Diagnosed with Chronic Myeloid Leukaemia. Br J Clin Pharmacol 88(4):1913–1924
(NCCN) NCCN. NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid Leukemia (Version 3.2022). available at http://www.nccn.org/patients. Accessed 27 Jan 2022;
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Petain A, Kattygnarath D, Azard J et al (2008) Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin Cancer Res 14(21):7102–7109
Wang Q, Jiang ZP, Yu EQ et al (2019) Population pharmacokinetic and pharmacogenetics of imatinib in Chinese patients with chronic myeloid leukemia. Pharmacogenomics 20(4):251–260
Eechoute K, Fransson MN, Reyners AK et al (2012) A long-term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res 18(20):5780–5787
Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR (2017) practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther 102(5):765–776
Widmer N, Bardin C, Chatelut E et al (2014) Review of therapeutic drug monitoring of anticancer drugs part two–targeted therapies. Eur J Cancer 50(12):2020–2036
Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD (2014) Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet 53(4):305–325
Widmer N, Decosterd LA, Csajka C et al (2006) Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol 62(1):97–112
Adeagbo BA, Olugbade TA, Durosinmi MA, Bolarinwa RA, Ogungbenro K, Bolaji OO (2017) Population Pharmacokinetics of imatinib in nigerians with chronic myeloid leukemia: clinical implications for dosing and resistance. J Clin Pharmacol 57(12):1554–1563
Shriyan B, Mehta P, Patil A et al (2022) Role of ADME gene polymorphisms on imatinib disposition: results from a population pharmacokinetic study in chronic myeloid leukaemia. Eur J Clin Pharmacol 78(8):1321–1330
Di Paolo A, Polillo M, Capecchi M et al (2014) The c480C>G polymorphism of hOCT1 influences imatinib clearance in patients affected by chronic myeloid leukemia. Pharmacogenomics J 14(4):328–335
Golabchifar AA, Rezaee S, Dinan NM, Kebriaeezadeh A, Rouini MR (2016) Population pharmacokinetic analysis of the oral absorption process and explaining intra-subject variability in plasma exposures of imatinib in healthy volunteers. Eur J Drug Metab Pharmacokinet 41(5):527–539
Renard D, Bouillon T, Zhou P, Flesch G, Quinn D (2015) Pharmacokinetic interactions among imatinib, bosentan and sildenafil, and their clinical implications in severe pulmonary arterial hypertension. Br J Clin Pharmacol 80(1):75–85
Schmidli H, Peng B, Riviere GJ et al (2005) Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 60(1):35–44
Judson I, Ma P, Peng B et al (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time EORTC soft tissue and bone sarcoma group. Cancer Chemother Pharmacol 55(4):379–386
Kretz O, Weiss HM, Schumacher MM, Gross G (2004) In vitro blood distribution and plasma protein binding of the tyrosine kinase inhibitor imatinib and its active metabolite, CGP74588, in rat, mouse, dog, monkey, healthy humans and patients with acute lymphatic leukaemia. Br J Clin Pharmacol 58(2):212–216
Prenen H, Guetens G, De Boeck G, Highley M, van Oosterom AT, de Bruijn EA (2006) Everolimus alters imatinib blood partition in favour of the erythrocyte. J Pharm Pharmacol 58(8):1063–1066
Gibbons J, Egorin MJ, Ramanathan RK et al (2008) Phase I and pharmacokinetic study of imatinib mesylate in patients with advanced malignancies and varying degrees of renal dysfunction: a study by the National Cancer Institute Organ Dysfunction Working Group. J Clin Oncol 26(4):570–576
Delbaldo C, Chatelut E, Ré M et al (2006) Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Can Res 12(20 Pt 1):6073–6078
Demetri GD, Wang Y, Wehrle E et al (2009) Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 27(19):3141–3147
Yeung CK, Shen DD, Thummel KE, Himmelfarb J (2014) Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int 85(3):522–528
Lalande L, Charpiat B, Leboucher G, Tod M (2014) Consequences of renal failure on non-renal clearance of drugs. Clin Pharmacokinet 53(6):521–532
Miners JO, Yang X, Knights KM, Zhang L (2017) The role of the kidney in drug elimination: transport, metabolism, and the impact of kidney disease on drug clearance. Clin Pharmacol Ther 102(3):436–449
Menon-Andersen D, Mondick JT, Jayaraman B et al (2009) Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol 63(2):229–238
Ansari M, Kalantary-Khandani B, Pardakhty A, Safavi M, Mosavi N, Mohajeri E (2016) Population Pharmacokinetics of Imatinib and its application to the therapeutic drug monitoring: Middle East CML population. Gulf J Oncolog 1(22):26–36
Gotta V, Bouchet S, Widmer N et al (2014) Large-scale imatinib dose-concentration-effect study in CML patients under routine care conditions. Leuk Res 38(7):764–772
Dalle Fratte C, Polesel J, Gagno S et al (2023) Impact of ABCG2 and ABCB1 polymorphisms on imatinib plasmatic exposure: an original work and meta-analysis. Int J Mol Sci 24(4):3303
Smith SA, Waters NJ (2019) Pharmacokinetic and Pharmacodynamic considerations for drugs binding to alpha-1-Acid glycoprotein. Pharma Res. https://doi.org/10.1007/s11095-018-2551-x
Funding
This work was supported by the Beijing Municipal Natural Science Foundation (grant number 7192218).
Author information
Authors and Affiliations
Contributions
LH and QJ made contributions to the conception and design. JZ and JB helped with the acquisition of data. BL performed imatinib quantification in plasma. LH and XH performed the extraction of genomic DNA and genotyping. SH and HH established the population pharmacokinetic model. SH and QS performed the statistical analysis and interpreted the data. SH wrote the manuscript. YL and YZ drew the tables. LH and QJ were involved in revising the manuscript critically for important intellectual content. All authors gave the final approval of the version to be published.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
This study was conducted following the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Peking University People’s Hospital (2022PHB095-001).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, S., Shao, Q., Zhao, J. et al. Population pharmacokinetics and pharmacogenetics analyses of imatinib in Chinese patients with chronic myeloid leukemia in a real-world situation. Cancer Chemother Pharmacol 92, 399–410 (2023). https://doi.org/10.1007/s00280-023-04581-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04581-0