Abstract
Purpose
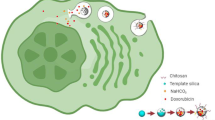
This study aims at chemotherapy and starvation therapy of HCC via starvation and apoptosis.
Methods
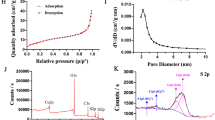
Hollow mesoporous organosilica nanoparticles (HMONs) with the thioether-hybrid structure were developed using an organic/inorganic co-templating assembly approach. Hydrofluoric acid was used to remove the internal MSN core for yielding large radial mesopores for loading drug cargos. The morphology and structure of NPs were determined using TEM and SEM. HMONs were stepwise surface modified with glucose oxidase (GOx), oxygen (O2) and Doxorubicin (DOX), and cancer cell membrane (CCM) for yielding CCM-coated HMONs (targeted stealth biorobots; TSBRs) for starvation, apoptotic, and enhanced cell uptake properties, respectively. The surface area and pore size distribution were determined via BET and BJH assays. The catalytic ability of GOx-modified NPs was measured using in vitro glucose conversion approach authenticated by H2O2 and pH determination assays. MTT assay was used to determine the cytotoxicities of NPs. Cell uptake and apoptotic assay were used for the NPs internalization and apoptosis mechanisms. The subcutaneous HepG2 tumor model was established in mice. The long-term in vivo toxicity was determined using blood assays.
Results
The prepared NPs were spherical, hollow and mesoporous with excellent surface area and pore size distribution. The GOx-modified NPs exhibited excellent catalytic activity. The TSBRs showed better cytotoxicity and reduce the tumor size and weight. The NPs showed long-term safety in vivo.
Conclusion
TSBRs destroyed cancer cells by starvation and chemotherapy in both in-vitro and in-vivo settings which demonstrates its anti-cancer potential.












Similar content being viewed by others
Data Availability
Data will be available on request.
References
Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48.
Park JW, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–66.
Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Llovet JM, et al. Hepatocellular carcinoma, (in eng). Nat Rev Dis Primers. 2016;2:16018.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
Devita VT Jr, Young RC, Canellos GP. Combination versus single agent chemotherapy: a review of the basis for selection of drug treatment of cancer. Cancer. 1975;35(1):98–110.
Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53(1):615–27.
Zhang Z, Wang J, Chen C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv Mater. 2013;25(28):3869–80.
Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10(4):853.
Ye B-L, Zheng R, Ruan X-J, Zheng Z-H, Cai H-J. Chitosan-coated doxorubicin nano-particles drug delivery system inhibits cell growth of liver cancer via p53/PRC1 pathway. Biochem Biophys Res Commun. 2018;495(1):414–20.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–70.
Tan Y, et al. In vivo programming of tumor mitochondria-specific doxorubicin delivery by a cationic glycolipid polymer for enhanced antitumor activity. Polym Chem. 2019;10(4):512–25.
Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–41.
Fu L-H, Qi C, Lin J, Huang P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem Soc Rev. 2018;47(17):6454–72.
Zhao W, Hu J, Gao W. Glucose oxidase–polymer nanogels for synergistic cancer-starving and oxidation therapy. ACS Appl Mater Interfaces. 2017;9(28):23528–35.
Zhou J, et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano. 2018;12(3):2858–72.
Fan W, et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed. 2017;56(5):1229–33.
Liu L, et al. An electrochemical biosensor with dual signal outputs: toward simultaneous quantification of pH and O2 in the brain upon ischemia and in a tumor during cancer starvation therapy. Angew Chem Int Ed. 2017;56(35):10471–5.
Birsoy K, et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508(7494):108–12.
Wu D, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–41.
Qu Q, Ma X, Zhao Y. Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers. Nanoscale. 2015;7(40):16677–86.
Sun L, et al. Real-time imaging of single-molecule enzyme cascade using a DNA origami raft. J Am Chem Soc. 2017;139(48):17525–32.
Gross AJ, Chen X, Giroud F, Travelet C, Borsali R, Cosnier S. Redox-active glyconanoparticles as electron shuttles for mediated electron transfer with bilirubin oxidase in solution. J Am Chem Soc. 2017;139(45):16076–9.
Koh MY, et al. A new HIF-1α/RANTES-driven pathway to hepatocellular carcinoma mediated by germline haploinsufficiency of SART 1/HAF in mice. Hepatology. 2016;63(5):1576–91.
Kong L, Zhou Y, Bu H, Lv T, Shi Y, Yang J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Cancer Res. 2016;35(1):131.
Jia X, et al. Perfluoropentane-encapsulated hollow mesoporous prussian blue nanocubes for activated ultrasound imaging and photothermal therapy of cancer. ACS Appl Mater Interfaces. 2015;7(8):4579–88.
Chen Y, et al. Multifunctional mesoporous composite nanocapsules for highly efficient MRI-guided high-intensity focused ultrasound cancer surgery. Angew Chem Int Ed. 2011;50(52):12505–9.
Yang WJ, et al. Nanogel-incorporated injectable hydrogel for synergistic therapy based on sequential local delivery of combretastatin-A4 phosphate (CA4P) and doxorubicin (DOX). ACS Appl Mater Interfaces. 2018;10(22):18560–73.
Chen K, et al. Stimuli-responsive polymer-doxorubicin conjugate: Antitumor mechanism and potential as nano-prodrug. Acta Biomater. 2019;84:339–55.
Duan Z, et al. PEGylated multistimuli-responsive dendritic prodrug-based nanoscale system for enhanced anticancer activity. ACS Appl Mater Interfaces. 2018;10(42):35770–83.
Shao W, Liu X, Sun G, Hu X-Y, Zhu J-J, Wang L. Construction of drug–drug conjugate supramolecular nanocarriers based on water-soluble pillar [6] arene for combination chemotherapy. Chem Commun. 2018;54(68):9462–5.
Yu G, et al. Supramolecular polymer-based nanomedicine: high therapeutic performance and negligible long-term immunotoxicity. J Am Chem Soc. 2018;140(25):8005–19.
Yao H, et al. Ultrasound and redox-triggered morphology transitions of supramolecular self-assemblies with pH responsiveness for triple-controlled release. Langmuir. 2019;35(24):8045–51.
Yu Z, et al. A pre-protective strategy for precise tumor targeting and efficient photodynamic therapy with a switchable DNA/upconversion nanocomposite. Chem Sci. 2018;9(14):3563–9.
Bottini M, Rosato N, Bottini N. PEG-modified carbon nanotubes in biomedicine: current status and challenges ahead. Biomacromol. 2011;12(10):3381–93.
Ballou B, et al. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug Chem. 2007;18(2):389–96.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751.
Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18.
Fan J-B, Huang C, Jiang L, Wang S. Nanoporous microspheres: from controllable synthesis to healthcare applications. J Mater Chem B. 2013;1(17):2222–35.
Lee CH, Lo LW, Mou CY, Yang CS. Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug. Adv Func Mater. 2008;18(20):3283–92.
Kim MH, et al. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery, (in eng). ACS Nano. 2011;5(5):3568–76.
Wu M, Meng Q, Chen Y, Du Y, Zhang L, Li Y, Zhang L, Shi J. Large-pore ultrasmall mesoporous organosilica nanoparticles: micelle/precursor co-templating assembly and nuclear-targeted gene delivery. Adv Mater. 2015;27(2):215–22.
Li Y, et al. Highly ordered mesoporous tungsten oxides with a large pore size and crystalline framework for H2S sensing, (in eng). Angew Chem Int Ed Engl. 2014;53(34):9035–40.
Kobler J, Möller K, Bein T. Colloidal suspensions of functionalized mesoporous silica nanoparticles. ACS Nano. 2008;2(4):791–9.
Kobler J, Bein T. Porous Thin Films of Functionalized Mesoporous Silica Nanoparticles. ACS Nano. 2008;2(11):2324–2330.
Inagaki S, Guan S, Ohsuna T, Terasaki O. An ordered mesoporous organosilica hybrid material with a crystal-like wall structure, (in eng). Nature. 2002;416(6878):304–7.
Liu R, Zhao X, Wu T, Feng P. Tunable redox-responsive hybrid nanogated ensembles. J Am Chem Soc. 2008;130(44):14418–14419.
Luo Z, et al. Mesoporous silica nanoparticles end-capped with collagen: redox-responsive nanoreservoirs for targeted drug delivery, (in eng). Angew Chem Int Ed Engl. 2011;50(3):640–3.
Lu N, et al. Biodegradable hollow mesoporous organosilica nanotheranostics for mild hyperthermia-induced bubble-enhanced oxygen-sensitized radiotherapy. ACS Nano. 2018;12(2):1580–1591.
Pan L, et al. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J Am Chem Soc. 2012;134(13):5722–5725.
Wang X, et al. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound (HIFU), (in eng). Adv Mater. 2012;24(6):785–91.
Cai X, Jia X, Gao W, Zhang K, Ma M, Wang S, Zheng Y, Shi J, Chen H. A versatile nanotheranostic agent for efficient dual-mode imaging guided synergistic chemo-thermal tumor therapy. Adv Funct Mater 2015;25:2520–2529.
Li SY, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy, (in eng). ACS Nano. 2017;11(7):7006–18.
Chen K-J, et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy, (in eng). ACS Nano 2014;8(5):5105–5115.
Pei D, Buyanova M. Overcoming endosomal entrapment in drug delivery, (in eng). Bioconjug Chem. 2019;30(2):273–83.
Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater. 2014;26(1):435–451.
Chen Y-C, et al. Chemo-photothermal effects of doxorubicin/silica–carbon hollow spheres on liver cancer. RSC Adv. 2018;8(64):36775–36784. https://doi.org/10.1039/C8RA08538B.
Yu Z, Zhou P, Pan W, Li N, Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun. 2018;9(1):5044.
Acknowledgements
This work was financially supported by the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG18B, 2020LKSFG02E), and the grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-Level University Development Program (002-18120314, 002-18120311) and the Post-doctoral fund by Shantou university medical college, Shantou, Guangdong Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
All authors declare no conflict of interest. The authors declare no competing/financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ullah, A., Khan, M., Yibang, Z. et al. Hollow Mesoporous Silica Nanoparticles for Dual Chemo-starvation Therapy of Hepatocellular Carcinoma. Pharm Res 40, 2215–2228 (2023). https://doi.org/10.1007/s11095-023-03599-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03599-6