Abstract
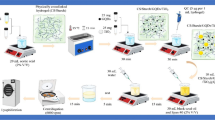
Improved treatment of acute myeloid leukemia (AML) could be possible by longer retention of anticancer drugs in the bloodstream. In this study, it was aimed to obtain improved treatment against AML by providing prolonged blood levels of doxorubicin and ensuring endosomal escape by the proton sponge effect. With this aim, pH-sensitive and chitosan-poly ethylene glycol (Cs-PEG) coated doxorubicin-loaded hollow mesoporous silica nanoparticles (C-HMSN-DN) were prepared. Nanoparticles (NPs) were characterized by dynamic light scattering (DLS), zeta potential, transmission electron microscopy (TEM), X-Ray diffraction (XRD), and nitrogen adsorption–desorption isotherms. High doxorubicin encapsulation efficacy was obtained as 90%. pH-sensitive formulations were showed higher cellular uptake and found more effective against human leukemia cell line (HL60) than non-pH sensitive formulations. In vivo studies showed that Cs-PEG coating prolonged blood circulation time tremendously in comparison to unmodified nanoparticles and free doxorubicin. The designed drug delivery system (DDS) can be more effective by endosomal escape to eliminate myeloid cells which are granular cells containing a great number of lysosomes. In conclusion, we present a drug delivery system that provides a prolonged blood circulation time due to Cs-PEG coating and effective drug delivery via pH-sensitive drug release and endosomal escape for AML treatment.
Graphical abstract








Similar content being viewed by others
References
Abumanhal-Masarweh H, Koren L, Zinger A, Yaari Z, Krinsky N, Kaneti G, Dahan N, Lupu-Haber Y, Suss-Toby E, Weiss-Messer E, Schlesinger-Laufer M, Shainsky-Roitman J, Schroeder A (2019) Sodium bicarbonate nanoparticles modulate the tumor pH and enhance the cellular uptake of doxorubicin. J Control Release 296:1–13
Bagwe RP, Hilliard LR, Tan W (2006) Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 22:4357–4362
Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M (2017) Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev 46:4218–4244
Cai D, Han C, Liu C, Ma X, Qian J, Zhou J, Li Y, Sun Y, Zhang C, Zhu W (2020) Chitosan-capped enzyme-responsive hollow mesoporous silica nanoplatforms for colon-specific drug delivery. Nanoscale Res Lett 15:123
Chen Y, Chen H, Guo L, He Q, Chen F, Zhou J, Feng J, Shi J (2010) Hollow/rattle-type mesoporous nanostructures by a structural difference-based selective etching strategy. ACS Nano 4:529–539
Chen Y, Yang W, Chang B, Hu H, Fang X, Sha X (2013) In vivo distribution and antitumor activity of doxorubicin-loaded N-isopropylacrylamide-co-methacrylic acid coated mesoporous silica nanoparticles and safety evaluation. Eur J Pharm Biopharm 85:406–412
Daeihamed M, Haeri A, Dadashzadeh S (2015) A simple and sensitive HPLC method for fluorescence quantitation of doxorubicin in micro-volume plasma: applications to pharmacokinetic studies in rats, Iran. J Pharm Res 14:33–42
Daryasari MP, Akhgar MR, Mamashli F, Bigdeli B, Khoobi M (2016) Chitosan-folate coated mesoporous silica nanoparticles as a smart and pH-sensitive system for curcumin delivery. RSC Adv 6:105578–105588
Fang X, Chen C, Liu Z, Liu P, Zheng N (2011) A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres. Nanoscale 3:1632–1639
Fang X, Zhao X, Fang W, Chen C, Zheng N (2013) Self-templating synthesis of hollow mesoporous silica and their applications in catalysis and drug delivery. Nanoscale 5:2205–2218
Feng W, Nie W, He C, Zhou X, Chen L, Qiu K, Wang W, Yin Z (2014) Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl Mater Interfaces 6:8447–8460
Fleischer CC, Payne CK (2014) Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes. Acc Chem Res 47:2651–2659
Gao Y, Chen Y, Ji X, He X, Yin Q, Zhang Z, Shi J, Li Y (2011) Controlled intracellular release of doxorubicin in multidrug-resistant cancer cells by tuning the shell-pore sizes of mesoporous silica nanoparticles. ACS Nano 5:9788–9798
García-Fernández A, Aznar E, Martínez-Máñez R, Sancenón F (2020) New advances in in vivo applications of gated mesoporous silica as drug delivery nanocarriers. Small 16:1902242
Glorani G, Marin R, Canton P, Pinto M, Conti G, Fracasso G, Riello P (2017) Pegylated silica nanoparticles: cytotoxicity and macrophage uptake. J Nanopart Res 19:294
Gunawan C, Lim M, Marquis CP, Amal R (2014) Nanoparticle-protein corona complexes govern the biological fates and functions of nanoparticles. J Mater Chem B 2:2060–2083
Han N, Zhao Q, Wan L, Wang Y, Gao Y, Wang P, Wang Z, Zhang J, Jiang T, Wang S (2015) Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS Appl Mater Interfaces 7:3342–3351
Herd H, Daum N, Jones AT, Huwer H, Ghandehari H, Lehr CM (2013) Nanoparticle geometry and surface orientation influence mode of cellular uptake. ACS Nano 7:1961–1973
Inocêncio S, Cordeiro T, Matos I, Danède F, Sotomayor JC, Fonseca IM, Correia NT, Corvo MC, Dionísio M (2021) Ibuprofen incorporated into unmodified and modified mesoporous silica: from matrix synthesis to drug release. Microporous Mesoporous Mater 310:110541
Kaasalainen M, Aseyev V, von Haartman E, Karaman DŞ, Mäkilä E, Tenhu H, Rosenholm J, Salonen J (2017) Size, stability, and porosity of mesoporous nanoparticles characterized with light scattering. Nanoscale Res Lett 12:74
Kanwal U, Irfan Bukhari N, Ovais M, Abass N, Hussain K, Raza A (2018) Advances in nano-delivery systems for doxorubicin: an updated insight. J Drug Target 26:296–310
Ke C-J, Su T-Y, Chen H-L, Liu H-L, Chiang W-L, Chu P-C, Xia Y, Sung H-W (2011) Smart multifunctional hollow microspheres for the quick release of drugs in intracellular lysosomal compartments. Angew Chem Int Ed 50:8086–8089
Krischke M, Hempel G, Völler S, André N, D’Incalci M, Bisogno G, Köpcke W, Borowski M, Herold R, Boddy AV, Boos J (2016) Pharmacokinetic and pharmacodynamic study of doxorubicin in children with cancer: results of a “European Pediatric Oncology Off-patents Medicines Consortium” trial. Cancer Chemother Pharmacol 78:1175–1184
Llopis-Lorente A, Lozano-Torres B, Bernardos A, Martínez-Máñez R, Sancenón F (2017) Mesoporous silica materials for controlled delivery based on enzymes. J Mater Chem B 5:3069–3083
Mekaru H, Lu J, Tamanoi F (2015) Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliv Rev 95:40–49
Pisani C, Gaillard JC, Odorico M, Nyalosaso JL, Charnay C, Guari Y, Chopineau J, Devoisselle JM, Armengaud J, Prat O (2017) The timeline of corona formation around silica nanocarriers highlights the role of the protein interactome. Nanoscale 9:1840–1851
Popat A, Liu J, Lu GQ, Qiao SZ (2012) A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J Mater Chem 22:11173–11178
Radu DR, Lai C-Y, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY (2004) A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc 126:13216–13217
Rawal M (2018) CHAPTER 2 Materials and chemistry of stimuli-responsive drug delivery systems, stimuli-responsive drug delivery systems. R Soc Chem 33–50
Robbins SL, Kumar V (2008) Robbins temel patoloji: (basic pathology), Nobel Tıp Kitabevi
Roca M, Haes AJ (2008) Silica−void−gold nanoparticles: temporally stable surface-enhanced Raman scattering substrates. J Am Chem Soc 130:14273–14279
Stewart BW, Wild C (2014) World cancer report 2014. International Agency for Research on Cancer, WHO Press, Lyon
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM (2016) PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 99:28–51
Sun Y-L, Yang B-J, Zhang SX-A, Yang Y-W (2012) Cucurbit [7] uril pseudorotaxane-based photoresponsive supramolecular nanovalve. Chem Eur J 18:9212–9216
Szegedi A, Popova M, Goshev I, Klébert S, Mihály J (2012) Controlled drug release on amine functionalized spherical MCM-41. J Solid State Chem 194:257–263
Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65:157–170
Teng Z, Su X, Zheng Y, Sun J, Chen G, Tian C, Wang J, Li H, Zhao Y, Lu G (2013) Mesoporous silica hollow spheres with ordered radial mesochannels by a spontaneous self-transformation approach. Chem Mater 98–105
Torchilin VP (2018) CHAPTER 1 Fundamentals of stimuli-responsive drug and gene delivery systems, stimuli-responsive drug delivery systems. R Soc Chem 1–32
Tuoriniemi J, Johnsson A-CJH, Holmberg JP, Gustafsson S, Gallego-Urrea JA, Olsson E, Pettersson JBC, Hassellöv M (2014) Intermethod comparison of the particle size distributions of colloidal silica nanoparticles. Sci Technol Adv Mater 15:035009
Varkouhi AK, Scholte M, Storm G, Haisma HJ (2011) Endosomal escape pathways for delivery of biologicals. J Control Release 151:220–228
Wang Z, Zhang X, Huang G, Gao J (2018) CHAPTER 3 pH-responsive drug delivery systems, stimuli-responsive drug delivery systems. R Soc Chem 51–82
Xiong L, Du X, Kleitz F, Qiao SZ (2015) Cancer-cell-specific nuclear-targeted drug delivery by dual-ligand-modified mesoporous silica nanoparticles. Small 11:5919–5926
Xu P, Zuo H, Chen B, Wang R, Ahmed A, Hu Y, Ouyang J (2017) Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep 7:42632
Yoo S, Lee J, Kim JM, Seong CY, Seong KD, Piao Y (2016) Well-dispersed sulfur wrapped in reduced graphene oxide nanoscroll as cathode material for lithium-sulfur battery. J Electroanal Chem 780:19–25
Zhao Q, Wang C, Liu Y, Wang J, Gao Y, Zhang X, Jiang T, Wang S (2014) PEGylated mesoporous silica as a redox-responsive drug delivery system for loading thiol-containing drugs. Int J Pharm 477:613–622
Zhu Y, Fang Y, Borchardt L, Kaskel S (2011) PEGylated hollow mesoporous silica nanoparticles as potential drug delivery vehicles. Microporous Mesoporous Mater 141:199–206
Acknowledgements
TEM, SEM, XRD, and BET analyses were performed in Middle East Technical University Central Laboratory. Special thanks to ILKO ARGEM for FTIR analysis. A part of this study was supported by Hacettepe University Coordinatorship of Scientific Research Projects with the project code THD-2016-12890. Special thanks to Prof. Fernandez-Megia and his team for giving Chitosan-PEG as a kind gift. Also, thanks to DEVA Holding A.S. for doxorubicin which was a kind gift from them.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ultav, G., Tonbul, H., Tavukcuoglu, E. et al. pH-sensitive chitosan-PEG-decorated hollow mesoporous silica nanoparticles could be an effective treatment for acute myeloid leukemia (AML). J Nanopart Res 24, 40 (2022). https://doi.org/10.1007/s11051-022-05404-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05404-8