Abstract
Introduction
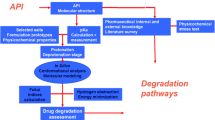
The formation of N-oxide degradants is a major concern in development of new drugs due to potential effects on a compound’s pharmacological activity. Such effects include but are not limited to solubility, stability, toxicity, and efficacy. In addition, these chemical transformations can impact physicochemical properties that affect drug manufacturability. Hence identification and control of N-oxide transformations is of critical importance in the development of new therapeutics.
Objective
This study describes the development of an in-silico approach to identify N-oxide formation in APIs with respect to autoxidation.
Methods
Average Local Ionization Energy (ALIE) calculations were carried out using molecular modeling techniques and application of Density Functional Theory (DFT) at the B3LYP/6-31G(d,p) level of theory. A total of 257 nitrogen atoms and 15 different oxidizable nitrogen types were used in developing this method.
Results
The results show that ALIE could be reliably used to predict the most susceptible nitrogen for N-oxide formation. A risk scale was developed that rapidly categorizes nitrogen’s oxidative vulnerabilities as small, medium, or high.
Conclusions
The developed process presents a powerful tool to identify structural susceptibilities for N-oxidation as well as enabling rapid structure elucidation in resolving potential experimental ambiguities.
Graphical Abstract










Similar content being viewed by others
References
Yoshioka S, Stella VJ. Stability of drugs and dosage forms. Springer Science & Business Media; 2000.
Kushwaha P. Significance of Stability Studies on Degradation Product. Res J Pharm Technol. 2009;2:621–7.
Rawat T, Pandey I. Forced degradation studies for drug substances and drug products-scientific and regulatory considerations. J Pharm Sci Res. 2015;7:238.
Niguram P, Kate AS. Structural characterization of forced degradation products of empagliflozin by high resolution mass spectrometry. J Liq Chromatogr Relat Technol. 2019;42:417–28.
Garg A, Solas DW, Takahashi LH, Cassella JV. Forced degradation of fentanyl: identification and analysis of impurities and degradants. J Pharm Biomed Anal. 2010;53:325–34.
Runje M, Babić S, Meštrović E, Nekola I, Dujmić-Vučinić Ž, Vojčić N. Forced degradation of nepafenac: Development and validation of stability indicating UHPLC method. J Pharm Biomed Anal. 2016;123:42–52.
Hartauer KJ, Arbuthnot GN, Baertschi SW, Johnson RA, Luke WD, Pearson NG, et al. Influence of peroxide impurities in povidone and crospovidone on the stability of raloxifene hydrochloride in tablets: identification and control of an oxidative degradation product. Pharm Dev Technol. 2000;5:303–10.
Bach RD, Su M-D, Schlegel HB. Oxidation of amines and sulfides with hydrogen peroxide and alkyl hydrogen peroxide. The nature of the oxygen-transfer step. J Am Chem Soc. 1994;116:5379–91.
Bäcktorp C, Örnskov E, Evertsson E, Remmelgas J, Broo A. A qualitative method for prediction of amine oxidation in methanol and water. J Pharm Sci. 2015;104:1409–20.
Voogd C, der Stel JV, Jacobs J. The mutagenic action of quindoxin, carbadox, olaquindox and some other N-oxides on bacteria and yeast. Mutat Res/Genet Toxicol. 1980;78:233–42.
Gabay M, Cabrera M, Maio RD, Paez JA, Campillo N, Lavaggi ML, et al. Mutagenicity of N-oxide containing heterocycles and related compounds: experimental and theoretical studies. Curr Top Med Chem. 2014;14:1374–87.
Tennant RW, Ashby J. Classification according to chemical structure, mutagenicity to Salmonella and level of carcinogenicity of a further 39 chemicals tested for carcinogenicity by the US National Toxicology Program. Mutat Res/Rev Genet Toxicol. 1991;257:209–27.
Pracht P, Wilcken R, Udvarhelyi A, Rodde S, Grimme S. High accuracy quantum-chemistry-based calculation and blind prediction of macroscopic pKa values in the context of the SAMPL6 challenge. J Comput Aided Mol Des. 2018;32:1139–49.
Bartolini M, Bertucci C, Gotti R, Tumiatti V, Cavalli A, Recanatini M, et al. Determination of the dissociation constants (pKa) of basic acetylcholinesterase inhibitors by reversed-phase liquid chromatography. J Chromatogr A. 2002;958:59–67.
Nielsen JE, Gunner M, Bertrand García-Moreno E. The pKa Cooperative: A collaborative effort to advance structure-based calculations of pKa values and electrostatic effects in proteins. Proteins. 2011;79:3249–59.
Uudsemaa M, Kanger T, Lopp M, Tamm T. pKa calculation for monoprotonated bipiperidine, bimorpholine and their derivatives in H2O and MeCN. Chem Phys Lett. 2010;485:83–6.
Toro-Labbé A. Theoretical aspects of chemical reactivity. Elsevier; 2006.
Politzer P, Murray JS, Bulat FA. Average local ionization energy: a review. J Mol Model. 2010;16:1731–42.
Kulshrestha P, Sukumar N, Murray JS, Giese RF, Wood TD. Computational prediction of antibody binding sites on tetracycline antibiotics: electrostatic potentials and average local ionization energies on molecular surfaces. J Phys Chem A. 2009;113:756–66.
Mary YS, Miniyar PB, Mary YS, Resmi K, Panicker CY, Armaković S, et al. Synthesis and spectroscopic study of three new oxadiazole derivatives with detailed computational evaluation of their reactivity and pharmaceutical potential. J Mol Struct. 2018;1173:469–80.
Brinck T, Stenlid JH. The molecular surface property approach: a guide to chemical interactions in chemistry, medicine, and material science. Adv Theory and Simul. 2019;2:1800149.
Hennemann M, Friedl A, Lobell M, Keldenich J, Hillisch A, Clark T, et al. CypScore: Quantitative prediction of reactivity toward cytochromes P450 based on semiempirical molecular orbital theory. ChemMedChem. 2009;4:657–69.
Politzer P, Lane P, Murray JS. Some interesting aspects of N-oxides. Mol Phys. 2014;112:719–25.
Chang G, Guida WC, Still WC. An internal-coordinate Monte Carlo method for searching conformational space. J Am Chem Soc. 1989;111:4379–86.
Saunders M, Houk K, Wu YD, Still WC, Lipton M, Chang G, et al. Conformations of cycloheptadecane. A comparison of methods for conformational searching. J Am Chem Soc. 1990;112:1419–27.
Mohamadi F, Richards NG, Guida WC, Liskamp R, Lipton M, Caufield C, et al. Macromodel—an integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J Comput Chem. 1990;11:440–67.
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, et al. Gaussian 16. 2016
Hadidi S, Shiri F, Norouzibazaz M. A DFT study of the degradation mechanism of anticancer drug carmustine in an aqueous medium. Struct Chem. 2019;30:1315–21.
Lin W, Zhao B, Ping S, Zhang X, Ji Y, Ren Y. Ultraviolet oxidative degradation of typical antidepressants: Pathway, product toxicity, and DFT theoretical calculation. Chemosphere. 2022;305: 135440.
Li Y, Yang Y, Lei J, Liu W, Tong M, Liang J. The degradation pathways of carbamazepine in advanced oxidation process: A mini review coupled with DFT calculation. Sci Total Environ. 2021;779: 146498.
Secretan P-H, Yayé HS, Sogaldi A, Antignac M, Tortolano L, Thirion O, et al. Intrinsic stability of the antiviral drug umifenovir by stress testing and DFT studies. J Pharm Biomed Anal. 2021;196: 113934.
Kurmi M, Singh S. Stability behavior of antiretroviral drugs and their combinations. 7: Comparative degradation pathways of lamivudine and emtricitabine and explanation to their differential degradation behavior by density functional theory. J Pharm Biomed Anal. 2017;142:155–61.
Feng L, Song W, Oturan N, Karbasi M, van Hullebusch ED, Esposito G, et al. Electrochemical oxidation of Naproxen in aqueous matrices: Elucidating the intermediates’ eco-toxicity, by assessing its degradation pathways via experimental and density functional theory (DFT) approaches. Chem Eng J. 2023;451: 138483.
Becke AD. Becke’s three parameter hybrid method using the LYP correlation functional. J Chem Phys. 1993;98:5648–52.
Pietro WJ, Francl MM, Hehre WJ, DeFrees DJ, Pople JA, Binkley JS. Self-consistent molecular orbital methods. 24. Supplemented small split-valence basis sets for second-row elements. J Am Chem Soc. 1982;104:5039–48.
Lu T, Chen F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J Mol Graph Model. 2012;38:314–23.
Brandes B, Halz JH, Merzweiler K, Deigner H-P, Csuk R. Synthesis and structure of azelastine-N-oxides. J Mol Struct. 2022;1251: 132033.
Wang Y, Yin H, Tang X, Wu Y, Meng Q, Gao Z. A series of cinchona-derived N-oxide phase-transfer catalysts: application to the photo-organocatalytic enantioselective α-hydroxylation of β-dicarbonyl compounds. J Org Chem. 2016;81:7042–50.
Shiomi N, Yamamoto K, Nagasaki K, Hatanaka T, Funahashi Y, Nakamura S. Enantioselective Oxidative Ring-Opening Reaction of Aziridines with α-Nitroesters Using Cinchona Alkaloid Amide/Nickel (II) Catalysts. Org Lett. 2017;19:74–7.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valdivia-Berroeta, G.A., Gonnella, N.C. N-oxidation Regioselectivity and Risk Prediction Using DFT-ALIE Calculations. Pharm Res 40, 1873–1883 (2023). https://doi.org/10.1007/s11095-023-03553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03553-6