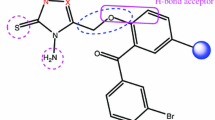
Abstract
The studied compound was synthesized by the reaction of diazotized 4-Aminobenzene and 2-amino-4-(4-aminophenyl) thiophene-3-carbonitrile (AATC). The synthesized structure was experimentally characterized using FT-IR and GC–MS followed by detailed computational investigations of the experimental structure and a subsequent application for molecular docking against the InhA receptors. The density functional theory (DFT) and time-dependent DFT calculations were performed using the B3LYP/6–311++G(d,p) method. The experimental vibrational frequencies were compared with the theoretical vibrational wavenumbers and the entire vibrational assignments were made solely to characterize the potential energy distributions (PED) using the Veda04 programme. The Fukui functions, MEP, and ADCH analysis were used to describe the reactive sites of the studied structure. Conceptual density functional theory (CDFT) and Frontier molecular orbitals (FMO) were used to characterize the reactivity parameters of the whole molecule while the aromaticity index was used to identify the most aromatic fragment of the studied compound. Natural bond orbital (NBO) analysis aided in describing the interactions that lead to the highest stabilization of the molecule and the interactions that undergo Intra- and Intermolecular charge transfer. Nonlinear optical property (NLOP) of APTC was also studied in order to ascertain its importance in technology such as optoelectronic devices. The Molecular docking studies were used to study the inhibitory action of the studied structure against some InhA proteins [an NADH-dependent enoyl-acyl carrier protein reductase (enoyl-ACP reductase)] using Auto-Dock vina software. The absorption, distribution, metabolism, excretion, and toxicity (ADMET) analysis was carried out using pq SCM to compare the drug physicochemical properties with standard drug isoniazid.





Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Hashemi SH, Kaykhaii M (2022) Azo dyes: sources, occurrence, toxicity, sampling, analysis, and their removal methods. Emerging freshwater pollutants. Elsevier, Amsterdam, pp 267–287
Gürses A, Açıkyıldız M, Günes K, Gürses MS (2016) Classification of dye and pigments. Dyes and pigments. Springer, New York, pp 31–45
Yao LW, Khan FSA, Mubarak NM, Karri RR, Khalid M, Walvekar R et al (2022) Insight into immobilization efficiency of Lipase enzyme as a biocatalyst on the graphene oxide for adsorption of Azo dyes from industrial wastewater effluent. J Mol Liq 354:118849
Selvaraj V, Karthika TS, Mansiya C, Alagar M (2021) An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J Mol Struct 1224:129195
Kapoor RT, Danish M, Singh RS, Rafatullah M, Hps AK (2021) Exploiting microbial biomass in treating azo dyes contaminated wastewater: mechanism of degradation and factors affecting microbial efficiency. J Water Process Eng 43:102255
Dulo B, Phan K, Githaiga J, Raes K, De Meester S (2021) Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste Biomass Valorization 12(12):6339–6374
Shankarling GS, Deshmukh PP, Joglekar AR (2017) Process intensification in azo dyes. J Environ Chem Eng 5(4):3302–3308
Pedgaonkar GS, Sridevi JP, Jeankumar VU, Saxena S, Devi PB, Renuka J, Sriram D (2014) Development of 2-(4-oxoquinazolin-3 (4H)-yl) acetamide derivatives as novel enoyl-acyl carrier protein reductase (InhA) inhibitors for the treatment of tuberculosis. Eur J Med Chem 86:613–627
Freundlich JS, Wang F, Vilchèze C, Gulten G, Langley R, Schiehser GA et al (2009) Triclosan derivatives: towards potent inhibitors of drug-sensitive and drug-resistant Mycobacterium tuberculosis. ChemMedChem 4(2):241
Romano E, Castillo MV, Pergomet JL, Zinczuk J, Brandán SA (2012) Synthesis and structural and vibrational analysis of (5, 7-dichloro-quinolin-8-yloxy) acetic acid. J Mol Struct 1018:149–155
Frisch MJ (2009) Gaussian09. http://www.gaussian.com/
Xu X, Goddard WA (2004) The X3LYP extended density functional for accurate descriptions of nonbond interactions, spin states, and thermochemical properties. Proc Natl Acad Sci 101(9):2673–2677
Karabulut E (2018) Linear reaction of Ca atom with HCl molecule in the ground electronic state: Hartree-Fock method. J Phys Chem Funct Mater 1(2):43–48
Jamroz MH (2004) Vibrational energy distribution analysis VEDA 4
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Robbins D, Newton AF, Gignoux C, Legeay JC, Sinclair A, Rejzek M et al (2011) Synthesis of natural-product-like scaffolds in unprecedented efficiency via a 12-fold branching pathway. Chem Sci 2(11):2232–2235
Murugavel S, Kannan D, Bakthadoss M (2017) Synthesis of a novel methyl (2E)-2-{[N-(2-formylphenyl)(4-methylbenzene) sulfonamido] methyl}-3-(2-methoxyphenyl) prop-2-enoate: Molecular structure, spectral, antimicrobial, molecular docking and DFT computational approaches. J Mol Struct 1127:457–475
Pires DE, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 58(9):4066–4072
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4(1):1–17
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723
Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM et al (2010) Function of human Rh based on structure of RhCG at 2.1 Å. Proc Natl Acad Sci 107(21):9638–9643
Fakih TM, Dewi ML (2020) In silico identification of characteristics spike glycoprotein of SARS-CoV-2 in the development novel candidates for COVID-19 infectious diseases. J Biomed Transl Res 6(2):48–52
Shepard RA (2021) A Computational investigation of cathode materials for next-generation secondary batteries (Doctoral dissertation, State University of New York at Binghamton)
Abul-Futouh H, Abaalkhail SJ, Harb MK, Görls H, Weigand W (2021) Structural studies and electrochemical catalysis investigation of [FeFe]-hydrogenase H-cluster mimics mediated by monophosphane ligands. Polyhedron 207:115382
Siebert S, Döll P (2010) Quantifying blue and green virtual water contents in global crop production as well as potential production losses without irrigation. J Hydrol 384(3–4):198–217
Krishnakumar V, Jayamani N, Mathammal R, Parasuraman K (2009) Density functional theory calculations and vibrational spectra of 2-bromo-4-chloro phenol and 2-chloro-4-nitro phenol. J Raman Spectrosc 40(11):1551–1556
Singho ND, Lah NAC, Johan MR, Ahmad R (2012) FTIR studies on silver-poly (methylmethacrylate) nanocomposites via in-situ polymerization technique. Int J Electrochem Sci 7(6):5596–5603
Vennila P, Govindaraju M, Venkatesh G, Kamal C (2016) Molecular structure, vibrational spectral assignments (FT-IR and FT-RAMAN), NMR, NBO, HOMO-LUMO and NLO properties of O-methoxybenzaldehyde based on DFT calculations. J Mol Struct 1111:151–156
Bansal A, Singh R, Singh RV (2000) Toxicity, spectroscopic characterization and electrochemical behaviour of new macrocclic complexes of lead (II) and palladium (II) metals. Met-Based Drugs 7(4):211–218
Wang JC, Wang PY, Huang RR, Lin WC, Fang CH, Pai LM, Nee TE (2011) Lie group study of Raman spectra of the Gurken gradient in Drosophila oogenesis. Anal Bioanal Chem 400(2):335–341
Rajesh AMMVP, Prabakaran AR, Gunasekaran S (2019) Extraction, Spectroscopic study of molecular structure and density functional theory studies Cis-vaccenic Acid from the leaves of Boswellia Serrata
Schwenke DW, Truhlar DG (1985) Systematic study of basis set superposition errors in the calculated interaction energy of two HF molecules. J Chem Phys 82(5):2418–2426
Gutowski M, van Duijneveldt-van de Rijdt JG, van Lenthe JH, van Duijneveldt FB (1993) Accuracy of the Boys and Bernardi function counterpoise method. J Chem Phys 98(6):4728–4737
Agwupuye JA, Louis H, Unimuke TO, David P, Ubana EI, Moshood YL (2021) Electronic structure investigation of the stability, reactivity, NBO analysis, thermodynamics, and the nature of the interactions in methyl-substituted imidazolium-based ionic liquids. J Mol Liq 337:116458
de Carvalho Marques Dourado JM (2021). Development and study of aminocatalyzed asymmetric organic reactions
Robb KA (2019) Enantioselective, Lewis base-catalyzed transformations: I. Polyene sulfenocyclization (preparative and mechanistic aspects) II. Sulfenofunctionalization of alkenyl boronates enabled by 1, 2-boronate migration (Doctoral dissertation, University of Illinois at Urbana-Champaign).
Karni O, Barré E, Pareek V, Georgaras JD, Man MK, Sahoo C et al (2022) Structure of the moiré exciton captured by imaging its electron and hole. Nature 603(7900):247–252
Yu C, Saalmann U, Rost JM (2022) High-order harmonics from backscattering of delocalized electrons. Phys Rev A 105(4):L041101
Mukherjee A, Lal S (2022) Superconductivity from repulsion in the doped 2D electronic Hubbard model: an entanglement perspective. J Phys: Condens Matter 34(27):275601
Solomatina AI, Kuznetsov KM, Gurzhiy VV, Pavlovskiy VV, Porsev VV, Evarestov RA, Tunik SP (2020) Luminescent organic dyes containing a phenanthro [9, 10-D] imidazole core and [Ir (N^ C)(N^ N)]+ complexes based on the cyclometalating and diimine ligands of this type. Dalton Trans 49(20):6751–6763
Kraner S, Prampolini G, Cuniberti G (2017) Exciton binding energy in molecular triads. J Phys Chem C 121(32):17088–17095
Pearson RG (1988) Electronic spectra and chemical reactivity. J Am Chem Soc 110(7):2092–2097
Benjamin I, Udoikono AD, Louis H, Agwamba EC, Unimuke TO, Owen AE, Adeyinka AS (2022) Antimalarial potential of naphthalene-sulfonic acid derivatives: Molecular electronic properties, vibrational assignments, and in-silico molecular docking studies. J Mol Struct 1264:133298
Agwamba EC, Udoikono AD, Louis H, Udoh EU, Benjamin I, Igbalagh AT et al (2022) Synthesis, characterization, DFT studies, and molecular modeling of azo dye derivatives as potential candidate for trypanosomiasis treatment. Chem Phys Impact 4:100076
Eno EA, Mbonu JI, Louis H, Patrick-Inezi FS, Gber TE, Unimke TO et al (2022) Antimicrobial activities of 1-phenyl-3-methyl-4-trichloroacetyl-pyrazolone: Experimental, DFT studies, and molecular docking investigation. J Indian Chem Soc 99:100524
Edet HO, Louis H, Benjamin I, Gideon M, Unimuke TO, Adalikwu SA, Nwagu AD, Adeyinka AS (2022) Hydrogen storage capacity of C12X12 (X= N, P, and Si). Chem Phys Impact 5:100107
Inah BE, Hitler L, Innocent B, Unimuke T, Adeyinka AS (2022) Computational study on the interactions of functionalized C24NC (NC= C-OH,-NH2,–COOH, and B) with chloroethylphenylbutanoic acid. Can J Chem. https://doi.org/10.1139/cjc-2022-0181
Gber TE, Louis H, Owen AE, Etinwa BE, Benjamin I, Asogwa FC et al (2022) Heteroatoms (Si, B, N, and P) doped 2D monolayer MoS 2 for NH 3 gas detection. RSC Adv 12(40):25992–26010
Louis H, Charlie DE, Amodu IO, Benjamin I, Gber TE, Agwamba EC, Adeyinka AS (2022) Probing the reactions of thiourea (CH4N2S) with Metals (X= Au, Hf, Hg, Ir, Os, W, Pt, and Re) Anchored on Fullerene Surfaces (C59X). ACS Omega 7:35118–35135
Makhlouf J, Louis H, Benjamin I, Ukwenya E, Valkonen A, Smirani W (2022) Single crystal investigations, spectral analysis, DFT studies, antioxidants, and molecular docking investigations of novel hexaisothiocyanato chromate complex. J Mol Struct 134223
Pedersen J, Mikkelsen KV (2022) A benchmark study of aromaticity indexes for benzene, pyridine and the diazines–I. Ground state aromaticity. RSC Adv 12(5):2830–2842
Manassir M, Pakiari AH (2022) Valence non-Lewis density as an approach to describe and measure aromaticity of organic and inorganic molecules. J Mol Graph Model 110:108062
Matito E, Salvador P, Duran M, Solà M (2006) Aromaticity measures from Fuzzy-Atom Bond Orders (FBO). The Aromatic fluctuation (FLU) and the para-delocalization (PDI) indexes. J Phys Chem A 110(15):5108–5113
Apebende CG, Idante PS, Louis H, Ameuru US, Unimuke TO, Gber TE et al (2022) Integrated spectroscopic, bio-active prediction and analytics of isoquinoline derivative for breast cancer mitigation. Chem Africa. https://doi.org/10.1007/s42250-022-00479-1
Agwamba EC, Louis H, Benjamin I, Apebende CG, Unimuke TO, Edet HO et al (2022) (E)-2-((3-nitrophenyl) diazenyl)-3-oxo-3-phenylpropanal: experimental, DFT studies, and molecular docking investigations. Chem Africa. https://doi.org/10.1007/s42250-022-00468-4
Benjamin I, Gber TE, Louis H, Ntui TN, Oyo-Ita EI, Unimuke TO et al (2022) Modelling of aminothiophene-carbonitrile derivatives as potential drug candidates for hepatitis B and C. Iran J Sci Technol Trans A Sci. https://doi.org/10.1007/s40995-022-01355-w
Clavian LM, Kumar KA, Rao DN, Shihab NK, Sanjeev G, Kumar PR (2022) Influence of structural and morphological features of zinc (II)-tetraphenylporphyrin thin film on its third order optical nonlinearity at pico and nano second regimes. J Lumin 246:118835
Qian J, Feng Z, Fan X, Kuzmin A, Gomes AS, Prasad PN (2022) High contrast 3-D optical bioimaging using molecular and nanoprobes optically responsive to IR light. Phys Rep 962:1–107
Semin S, Li X, Duan Y, Rasing T (2021) Nonlinear optical properties and applications of fluorenone molecular materials. Adv Opt Mater 9(23):2100327
Mohanraj P, Sivakumar R, Massoud EES (2022) Role of higher order dispersion on instability criterion of saturable fiber system with non-Kerr nonlinearities. Optik 253:168608
Al-Sulami AI, Basha MT, Althagafy HS, Al-Zaydi KM, Davaasuren B, Al-kaff NS, Said MA (2022) Azo-based multifunctional molecules and their copper (II) complexes as potential inhibitors against Alzheimer’s disease: XRD/Hirshfeld analysis/DFT/molecular docking/cytotoxicity. Inorg Chem Commun 142:109535
Stigliani JL, Bernardes-Génisson V, Bernadou J, Pratviel G (2012) Cross-docking study on InhA inhibitors: a combination of Autodock Vina and PM6-DH2 simulations to retrieve bio-active conformations. Org Biomol Chem 10(31):6341–6349
Acknowledgements
The authors are thankful to Dr. J.E. Ishegbe of the Department of Polymer and Textile Engineering, Ahmadu Bello University for the method of synthesis, characterization, and spectroscopic analysis.
Funding
This research work was not funded by any external funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare in unity zero conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
42250_2022_544_MOESM1_ESM.docx
The supporting information contains tables and figures showing ADCH, global and local reactivity descriptors and second-order perturbation analysis of NBO. (DOCX 25 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ntui, T.N., Oyo-Ita, E.E., Agwupuye, J.A. et al. Synthesis, Spectroscopic, DFT Study, and Molecular Modeling of Thiophene-Carbonitrile Against Enoyl-ACP Reductase Receptor. Chemistry Africa 6, 945–966 (2023). https://doi.org/10.1007/s42250-022-00544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00544-9