Abstract
Purpose
To develop a whole physiologically based pharmacokinetic-pharmacodynamic (PBPK-PD) model to describe the pharmacokinetics and anti-gastric acid secretion of omeprazole in CYP2C19 extensive metabolizers (EMs), intermediate metabolizers (IMs), poor metabolizers (PMs) and ultrarapid metabolizers (UMs) following oral or intravenous administration.
Methods
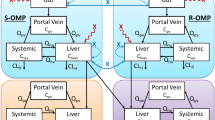
A PBPK/PD model was built using Phoenix WinNolin software. Omeprazole was mainly metabolized by CYP2C19 and CYP3A4 and the CYP2C19 polymorphism was incorporated using in vitro data. We described the PD by using a turn-over model with parameter estimates from dogs and the effect of a meal on the acid secretion was also implemented. The model predictions were compared to 53 sets of clinical data.
Results
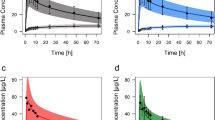
Predictions of omeprazole plasma concentration (72.2%) and 24 h stomach pH after administration (85%) were within 0.5–2.0-fold of the observed values, indicating that the PBPK-PD model was successfully developed. Sensitivity analysis demonstrated that the contributions of the tested factors to the plasma concentration of omeprazole were Vmax,2C19 ≈ Papp > Vmax,3A4 > Kti, and contributions to its pharmacodynamic were Vmax,2C19 > kome > kms > Papp > Vmax,3A4. The simulations showed that while the initial omeprazole dose in UMs, EMs, and IMs increased 7.5-, 3- and 1.25-fold compared to those of PMs, the therapeutic effect was similar.
Conclusions
The successful establishment of this PBPK-PD model highlights that pharmacokinetic and pharmacodynamic profiles of drugs can be predicted using preclinical data. The PBPK-PD model also provided a feasible alternative to empirical guidance for the recommended doses of omeprazole.






Similar content being viewed by others
Data Availability
All data selected during this study are included in this published article and its electronic supplementary information files, and further inquiries can be directed to the corresponding authors.
Abbreviations
- AUC:
-
Area under the curve
- Cmax :
-
Peak plasma concentrations
- EMs:
-
Extensive metabolizers
- IMs:
-
Intermediate metabolizers
- IVIVE:
-
In vitro to in vivo extrapolation
- Papp :
-
Apparent permeability
- Peff :
-
Effective permeability coefficient
- PBPK-PD:
-
Physiologically based pharmacokinetic-pharmacodynamic
- PMs:
-
Poor metabolizers
- PPI:
-
Proton pump inhibitor
- UMs:
-
Ultrarapid metabolizers
- VPCs:
-
Visual predictive checks
References
Howden CW, Holt S. Acid suppression as treatment for NSAID-related peptic ulcers. Am J Gastroenterol. 1991;86(12):1720–2.
Yamazaki H, Inoue K, Shaw PM, Checovich WJ, Guengerich FP, Shimada T. Different contributions of cytochrome P450 2C19 and 3A4 in the oxidation of omeprazole by human liver microsomes: effects of contents of these two forms in individual human samples. J Pharmacol Exp Ther. 1997;283(2):434–42.
Botton MR, Whirl-Carrillo M, Del Tredici AL, Sangkuhl K, Cavallari LH, Agúndez JAG, et al. PharmVar GeneFocus: CYP2C19. Clin Pharmacol Ther. 2021;109(2):352–66. https://doi.org/10.1002/cpt.1973.
Hicks JK, Bishop JR, Sangkuhl K, Müller DJ, Ji Y, Leckband SG, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127–34. https://doi.org/10.1002/cpt.147.
Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French registry of acute ST-elevation and non-ST-elevation myocardial infarction (FAST-MI) registry. Circ. 2011;123(5):474–82. https://doi.org/10.1161/circulationaha.110.965640.
Zhang HJ, Zhang XH, Liu J, Sun LN, Shen YW, Zhou C, et al. Effects of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of proton pump inhibitors. Pharmacol Res. 2020;152:104606. https://doi.org/10.1016/j.phrs.2019.104606.
Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol Ther. 2006;44(7):297–302. https://doi.org/10.5414/cpp44297.
Payan M, Rouini MR, Tajik N, Ghahremani MH, Tahvilian R. Hydroxylation index of omeprazole in relation to CYP2C19 polymorphism and sex in a healthy Iranian population. DARU : J Faculty Pharm Tehran Univ Med Sci. 2014;22(1):81. https://doi.org/10.1186/s40199-014-0081-6.
Bell NJ, Hunt RH. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut. 1992;33(1):118–24. https://doi.org/10.1136/gut.33.1.118.
Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterol. 1990;99(2):345–51. https://doi.org/10.1016/0016-5085(90)91015-x.
Howden CW, Hunt RH. The relationship between suppression of acidity and gastric ulcer healing rates. Aliment Pharmacol Ther. 1990;4(1):25–33. https://doi.org/10.1111/j.1365-2036.1990.tb00445.x.
Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19–31. https://doi.org/10.1007/s00228-008-0576-5.
Shimatani T, Inoue M, Kuroiwa T, Xu J, Mieno H, Nakamura M, et al. Acid-suppressive effects of rabeprazole, omeprazole, and lansoprazole at reduced and standard doses: a crossover comparative study in homozygous extensive metabolizers of cytochrome P450 2C19. Clin Pharmacol Ther. 2006;79(1):144–52. https://doi.org/10.1016/j.clpt.2005.09.012.
Zhou L, Gan J, Yoshitsugu H, Gu X, Lutz JD, Masson E, et al. Integration of physiologically-based pharmacokinetic modeling into early clinical development: An investigation of the pharmacokinetic nonlinearity. CPT Pharmacometrics Syst Pharmacol. 2015;4(5):286–94. https://doi.org/10.1002/psp4.35.
Chen Y, Jin JY, Mukadam S, Malhi V, Kenny JR. Application of IVIVE and PBPK modeling in prospective prediction of clinical pharmacokinetics: strategy and approach during the drug discovery phase with four case studies. Biopharm Drug Dispos. 2012;33(2):85–98. https://doi.org/10.1002/bdd.1769.
Scotcher D, Galetin A. PBPK simulation-based evaluation of ganciclovir Crystalluria risk factors: effect of renal impairment, old age, and low fluid intake. AAPS J. 2021;24(1):13. https://doi.org/10.1208/s12248-021-00654-1.
Chen Y, Zhao K, Liu F, Xie Q, Zhong Z, Miao M, et al. Prediction of Deoxypodophyllotoxin disposition in mouse, rat, monkey, and dog by physiologically based pharmacokinetic model and the extrapolation to human. Front Pharmacol. 2016;7:488. https://doi.org/10.3389/fphar.2016.00488.
Bhatnagar S, Mukherjee D, Salem AH, Miles D, Menon RM, Gibbs JP. Dose adjustment of venetoclax when co-administered with posaconazole: clinical drug-drug interaction predictions using a PBPK approach. Cancer Chemother Pharmacol. 2021;87(4):465–74. https://doi.org/10.1007/s00280-020-04179-w.
Abouir K, Samer CF, Gloor Y, Desmeules JA, Daali Y. Reviewing data integrated for PBPK model development to predict metabolic drug-drug interactions: shifting perspectives and emerging trends. Front Pharmacol. 2021;12:708299. https://doi.org/10.3389/fphar.2021.708299.
Kong WM, Sun BB, Wang ZJ, Zheng XK, Zhao KJ, Chen Y, et al. Physiologically based pharmacokinetic-pharmacodynamic modeling for prediction of vonoprazan pharmacokinetics and its inhibition on gastric acid secretion following intravenous/oral administration to rats, dogs and humans. Acta Pharmacol Sin. 2020;41(6):852–65. https://doi.org/10.1038/s41401-019-0353-2.
Fryklund J, Gedda K, Wallmark B. Specific labelling of gastric H+,K+-ATPase by omeprazole. Biochem Pharmacol. 1988;37(13):2543–9. https://doi.org/10.1016/0006-2952(88)90244-4.
Abelö A, Eriksson UG, Karlsson MO, Larsson H, Gabrielsson J. A turnover model of irreversible inhibition of gastric acid secretion by omeprazole in the dog. J Pharmacol Exp Ther. 2000;295(2):662–9.
Abelö A, Holstein B, Eriksson UG, Gabrielsson J, Karlsson MO. Gastric acid secretion in the dog: a mechanism-based pharmacodynamic model for histamine stimulation and irreversible inhibition by omeprazole. J Pharmacokinet Pharmacodyn. 2002;29(4):365–82. https://doi.org/10.1023/a:1020905224001.
Dujic T, Cvijic S, Elezovic A, Bego T, Imamovic Kadric S, Malenica M, et al. Interaction between omeprazole and Gliclazide in relation to CYP2C19 phenotype. J Personal Med. 2021;11(5) https://doi.org/10.3390/jpm11050367.
Wu F, Gaohua L, Zhao P, Jamei M, Huang SM, Bashaw ED, et al. Predicting nonlinear pharmacokinetics of omeprazole enantiomers and racemic drug using physiologically based pharmacokinetic modeling and simulation: application to predict drug/genetic interactions. Pharm Res. 2014;31(8):1919–29. https://doi.org/10.1007/s11095-013-1293-z.
Wang Z, Yang H, Xu J, Zhao K, Chen Y, Liang L, et al. Prediction of atorvastatin pharmacokinetics in high-fat diet and low-dose Streptozotocin-induced diabetic rats using a Semiphysiologically based pharmacokinetic model involving both enzymes and transporters. Drug Metab Dispos. 2019;47(10):1066–79. https://doi.org/10.1124/dmd.118.085902.
Marier JF, Dubuc MC, Drouin E, Alvarez F, Ducharme MP, Brazier JL. Pharmacokinetics of omeprazole in healthy adults and in children with gastroesophageal reflux disease. Ther Drug Monit. 2004;26(1):3–8. https://doi.org/10.1097/00007691-200402000-00003.
Cui C, Sun J, Wang X, Yu Z, Shi Y. Factors contributing to drug release from enteric-coated omeprazole capsules: An in vitro and in vivo pharmacokinetic study and IVIVC evaluation in beagle dogs. Dose-Response : Publ Int Hormesis Soc. 2020;18(1):1559325820908980. https://doi.org/10.1177/1559325820908980.
Xu RJ, Kong WM, An XF, Zou JJ, Liu L, Liu XD. Physiologically-based pharmacokinetic-pharmacodynamics model characterizing CYP2C19 polymorphisms to predict Clopidogrel pharmacokinetics and its anti-platelet aggregation effect following Oral administration to coronary artery disease patients with or without diabetes. Front Pharmacol. 2020;11:593982. https://doi.org/10.3389/fphar.2020.593982.
Westerhout J, van de Steeg E, Grossouw D, Zeijdner EE, Krul CA, Verwei M, et al. A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur J Pharm Sci Official J Eur Fed Pharma Sci. 2014;63:167–77. https://doi.org/10.1016/j.ejps.2014.07.003.
Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007;8(7):676–84. https://doi.org/10.2174/138920007782109733.
Rowland Yeo K, Jamei M, Yang J, Tucker GT, Rostami-Hodjegan A. Physiologically based mechanistic modelling to predict complex drug-drug interactions involving simultaneous competitive and time-dependent enzyme inhibition by parent compound and its metabolite in both liver and gut - the effect of diltiazem on the time-course of exposure to triazolam. Eur J Pharm Sci Official J Eur Fed Pharm Sci. 2010;39(5):298–309. https://doi.org/10.1016/j.ejps.2009.12.002.
Drozdzik M, Busch D, Lapczuk J, Müller J, Ostrowski M, Kurzawski M, et al. Protein abundance of clinically relevant drug-metabolizing enzymes in the human liver and intestine: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2018;104(3):515–24. https://doi.org/10.1002/cpt.967.
Furuta T, Ohashi K, Kosuge K, Zhao XJ, Takashima M, Kimura M, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65(5):552–61. https://doi.org/10.1016/s0009-9236(99)70075-5.
Berstad K, Massey H, Berstad A. Effect of enprostil on basal and meal-stimulated gastric acid and pepsin secretion, serum gastrin and gastric emptying in healthy persons. Aliment Pharmacol Ther. 1988;2(1):65–72. https://doi.org/10.1111/j.1365-2036.1988.tb00673.x.
Steere B, Baker JA, Hall SD, Guo Y. Prediction of in vivo clearance and associated variability of CYP2C19 substrates by genotypes in populations utilizing a pharmacogenetics-based mechanistic model. Drug Metabol Dispos Biol Fate Chem. 2015;43(6):870–83. https://doi.org/10.1124/dmd.114.061523.
Rowland Yeo K, Walsky RL, Jamei M, Rostami-Hodjegan A, Tucker GT. Prediction of time-dependent CYP3A4 drug-drug interactions by physiologically based pharmacokinetic modelling: impact of inactivation parameters and enzyme turnover. Eur J Pharm Sci. 2011;43(3):160–73. https://doi.org/10.1016/j.ejps.2011.04.008.
Qian CQ, Zhao KJ, Chen Y, Liu L, Liu XD. Simultaneously predict pharmacokinetic interaction of rifampicin with oral versus intravenous substrates of cytochrome P450 3A/P-glycoprotein to healthy human using a semi-physiologically based pharmacokinetic model involving both enzyme and transporter turnover. Eur J Pharm Sci. 2019;134:194–204. https://doi.org/10.1016/j.ejps.2019.04.026.
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79(1):103–13. https://doi.org/10.1016/j.clpt.2005.10.002.
Samant S, Jiang XL, Peletier LA, Shuldiner AR, Horenstein RB, Lewis JP, et al. Identifying clinically relevant sources of variability: the clopidogrel challenge. Clin Pharmacol Ther. 2017;101(2):264–73. https://doi.org/10.1002/cpt.459.
Schmitt W. General approach for the calculation of tissue to plasma partition coefficients. Toxicol in Vitro. 2008;22(2):457–67. https://doi.org/10.1016/j.tiv.2007.09.010.
Hanioka N, Tsuneto Y, Saito Y, Maekawa K, Sawada J, Narimatsu S. Influence of CYP2C19*18 and CYP2C19*19 alleles on omeprazole 5-hydroxylation: in vitro functional analysis of recombinant enzymes expressed in Saccharomyces cerevisiae. Basic Clin Pharmacol Toxicol. 2008;102(4):388–93. https://doi.org/10.1111/j.1742-7843.2008.00222.x.
Guo Y, Lucksiri A, Dickinson GL, Vuppalanchi RK, Hilligoss JK, Hall SD. Quantitative prediction of CYP3A4- and CYP3A5-mediated drug interactions. Clin Pharmacol Ther. 2020;107(1):246–56. https://doi.org/10.1002/cpt.1596.
Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedeberg's Arch Pharmacol. 2001;364(6):551–7. https://doi.org/10.1007/s00210-001-0489-7.
Nakamura T, Arai Y, Tando Y, Terada A, Yamada N, Tsujino M, et al. Effect of omeprazole on changes in gastric and upper small intestine pH levels in patients with chronic pancreatitis. Clin Ther. 1995;17(3):448–59. https://doi.org/10.1016/0149-2918(95)80110-3.
Capitanini A, Lupi A, Osteri F, Petrone I, Del Corso C, Straniti M, et al. Gastric pH, sevelamer hydrochloride and omeprazole. Clin Nephrol. 2005;64(4):320–2. https://doi.org/10.5414/cnp64320.
Uno T, Niioka T, Hayakari M, Yasui-Furukori N, Sugawara K, Tateishi T. Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol. 2007;63(2):143–9. https://doi.org/10.1007/s00228-006-0251-7.
Furuta K, Adachi K, Ohara S, Morita T, Tanimura T, Koshino K, et al. Relationship between the acid-inhibitory effects of two proton pump inhibitors and CYP2C19 genotype in Japanese subjects: a randomized two-way crossover study. J Int Med Res. 2010;38(4):1473–83. https://doi.org/10.1177/147323001003800430.
Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15(12):1929–37. https://doi.org/10.1046/j.1365-2036.2001.01108.x.
Shimatani T, Inoue M, Kuroiwa T, Horikawa Y, Mieno H, Nakamura M. Effect of omeprazole 10 mg on intragastric pH in three different CYP2C19 genotypes, compared with omeprazole 20 mg and lafutidine 20 mg, a new H2-receptor antagonist. Aliment Pharmacol Ther. 2003;18(11–12):1149–57. https://doi.org/10.1046/j.1365-2036.2003.01804.x.
Sahara S, Sugimoto M, Uotani T, Ichikawa H, Yamade M, Iwaizumi M, et al. Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38(9):1129–37. https://doi.org/10.1111/apt.12492.
Hu XP, Xu JM, Hu YM, Mei Q, Xu XH. Effects of CYP2C19 genetic polymorphism on the pharmacokinetics and pharmacodynamics of omeprazole in Chinese people. J Clin Pharm Ther. 2007;32(5):517–24. https://doi.org/10.1111/j.1365-2710.2007.00851.x.
Saitoh T, Fukushima Y, Otsuka H, Hirakawa J, Mori H, Asano T, et al. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther. 2002;16(10):1811–7. https://doi.org/10.1046/j.1365-2036.2002.01348.x.
Hunfeld NG, Mathot RA, Touw DJ, van Schaik RH, Mulder PG, Franck PF, et al. Effect of CYP2C19*2 and *17 mutations on pharmacodynamics and kinetics of proton pump inhibitors in Caucasians. Br J Clin Pharmacol. 2008;65(5):752–60. https://doi.org/10.1111/j.1365-2125.2007.03094.x.
Park S, Hyun YJ, Kim YR, Lee JH, Ryu S, Kim JM, et al. Effects of CYP2C19 genetic polymorphisms on PK/PD responses of omeprazole in Korean healthy volunteers. J Korean Med Sci. 2017;32(5):729–36. https://doi.org/10.3346/jkms.2017.32.5.729.
Chowers Y, Atarot T, Pratha VS, Fass R. The effect of once daily omeprazole and succinic acid (VECAM) vs once daily omeprazole on 24-h intragastric pH. Neurogastroenterol Motil Official J Eur Gastrointest Motil Soc. 2012;24(5):426–31–e208–9. https://doi.org/10.1111/j.1365-2982.2012.01884.x.
Shimatani T, Inoue M, Kuroiwa T, Xu J, Tazuma S, Horikawa Y, et al. Acid-suppressive efficacy of a reduced dosage of rabeprazole: comparison of 10 mg twice daily rabeprazole with 20 mg twice daily rabeprazole, 30 mg twice daily lansoprazole, and 20 mg twice daily omeprazole by 24-hr intragastric pH-metry. Dig Dis Sci. 2005;50(7):1202–6. https://doi.org/10.1007/s10620-005-2760-0.
Kim KN, Yang SW, Kim H, Kwak SS, Kim YS, Cho DY. Acid inhibitory effect of a combination of omeprazole and sodium bicarbonate (CDFR0209) compared with delayed-release omeprazole 40 mg alone in healthy adult male subjects. Clin Pharmacol Drug Dev. 2018;7(1):53–8. https://doi.org/10.1002/cpdd.331.
Roh HK, Kim PS, Lee DH, Tybring G, Sagar M, Park CS, et al. Omeprazole treatment of Korean patients: effects on gastric pH and gastrin release in relation to CYP2C19 geno- and phenotypes. Basic Clin Pharma Toxic. 2004;95(3):112–9. https://doi.org/10.1111/j.1742-7843.2004.950302.x.
Sagar M, Tybring G, Dahl ML, Bertilsson L, Seensalu R. Effects of omeprazole on intragastric pH and plasma gastrin are dependent on the CYP2C19 polymorphism. Gastroenterol. 2000;119(3):670–6. https://doi.org/10.1053/gast.2000.16515.
Miehlke S, Löbe S, Madisch A, Kuhlisch E, Laass M, Grossmann D, et al. Intragastric acidity during administration of generic omeprazole or esomeprazole - a randomised, two-way crossover study including CYP2C19 genotyping. Aliment Pharmacol Ther. 2011;33(4):471–6. https://doi.org/10.1111/j.1365-2036.2010.04544.x.
Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, Ishizaki T. Initial 48-hour acid inhibition by intravenous infusion of omeprazole, famotidine, or both in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2006;80(5):539–48. https://doi.org/10.1016/j.clpt.2006.08.010.
Wang Y, Zhang H, Meng L, Wang M, Yuan H, Ou N, et al. Influence of CYP2C19 on the relationship between pharmacokinetics and intragastric pH of omeprazole administered by successive intravenous infusions in Chinese healthy volunteers. Eur J Clin Pharmacol. 2010;66(6):563–9. https://doi.org/10.1007/s00228-010-0821-6.
Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41(12):913–58. https://doi.org/10.2165/00003088-200241120-00002.
Koukoula M, Dotsikas Y, Molou E, Schulpis KH, Thodi G, Chatzidaki M, et al. Study of the effect of CYP2C19 polymorphisms on omeprazole pharmacokinetics by utilizing validated LC-MS/MS and real time-PCR methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1047:173–9. https://doi.org/10.1016/j.jchromb.2016.06.046.
Andersson T, Bergstrand R, Cederberg C. Influence of acid secretory status on absorption of omeprazole from enteric coated granules. Br J Clin Pharmacol. 1991;31(3):275–8. https://doi.org/10.1111/j.1365-2125.1991.tb05530.x.
Nyrén O, Gustavsson S, Adami HO, Lööf L. Methodological aspects of clinical trials in non-ulcer dyspepsia with special reference to selectional factors. Scand J Gastroenterol Suppl. 1985;109:159–62. https://doi.org/10.3109/00365528509103952.
Tamminga WJ, Wemer J, Oosterhuis B, Weiling J, Wilffert B, de Leij LF, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55(3):177–84. https://doi.org/10.1007/s002280050615.
Ramsjö M, Aklillu E, Bohman L, Ingelman-Sundberg M, Roh HK, Bertilsson L. CYP2C19 activity comparison between swedes and Koreans: effect of genotype, sex, oral contraceptive use, and smoking. Eur J Clin Pharmacol. 2010;66(9):871–7. https://doi.org/10.1007/s00228-010-0835-0.
Xie HG, Huang SL, Xu ZH, Xiao ZS, He N, Zhou HH. Evidence for the effect of gender on activity of (S)-mephenytoin 4′-hydroxylase (CYP2C19) in a Chinese population. Pharmacogenet. 1997;7(2):115–9. https://doi.org/10.1097/00008571-199704000-00004.
Acknowledgments
We would like to thank all the authors for their participation and helpful discussions. Due to space constraints, we are aware that there is far more research associated with this field and regret that we could not cite every available report.
Funding
This study was supported by funding from the National Natural Science Foundation of China (N0.82073922; 82173884; 82204511), the “Double First-Class” university project (No. CPU2022QZ21) and the Jiangsu Funding Program for Excellent Postdoctoral Talent (No. 1412200067).
Author information
Authors and Affiliations
Contributions
SL, HY, LL, and XL designed the paper frame. LX, LJ, and YY selected the data. LY and HZ prepared the figures and tables. SL, LL, and XL wrote the manuscript. All authors contributed to the paper and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Xie, L., Yang, L. et al. Prediction of Omeprazole Pharmacokinetics and its Inhibition on Gastric Acid Secretion in Humans Using Physiologically Based Pharmacokinetic-Pharmacodynamic Model Characterizing CYP2C19 Polymorphisms. Pharm Res 40, 1735–1750 (2023). https://doi.org/10.1007/s11095-023-03531-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03531-y