Abstract
Purpose
Is the complexity of medical product (medicines and medical devices) regulation impacting innovation in the US? If so, how?
Methods
Here, this question is investigated as follows: Various novel proxy metrics of regulation (FDA-issued guidelines) and innovation (corresponding FDA-registrations) from 1976–2020 are used to determine interdependence, a concept relying on strong correlation and reciprocal causality (estimated via variable lag transfer entropy and wavelet coherence).
Results
Based on the observed interdependence, a mapping of regulation onto innovation is conducted and finds that regulation seems to accelerate then supports innovation until on or around 2015; at which time, an inverted U-curve emerged.
Conclusions
If empirically evidentiary, an important innovation-regulation nexus in the US has been reached; and, as such, stakeholders should (re)consider the complexity of the regulatory landscape to enhance US medical product innovation. Study limitations, extensions, and further thoughts complete this investigation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A prevailing discourse in markets relates to the impact of regulation onto innovation [1, 2]. This is of particular interest in United States (US) medical products industry, regulated under the Food and Drug Administration (FDA), as the regulatory landscape continues to rapidly evolve since the mid-1970s with the concomitant rise in the interest in global innovative competitiveness and accompanied legislation including the promulgation of guidelines [3–9].Footnote 1 The question of interest in this context is: Does US medical product regulation help or hinder innovation? In this work, it is hypothesized that (1) there is a relationship between regulation and innovation latent within US medical products,(2) that this relationship may be elucidated using novel, yet objective, proxy (surrogate) metrics; and (3) the relationship would give insight into the temporal impact of US medical product regulation onto innovation.
The following assumptions are used to build an infrastructure to test these hypotheses and thus provide insight into the overriding question:
-
(1)
FDA-registrations and FDA-issued guidelines may be used as surrogate metrics to investigate medical product innovation and regulation, respectively.
Innovation as the Number of FDA-Registered Medical Products
Onur and Söderberg [10] used “all product market approvals in the high-risk medical device market over the 1978–2007 period” – Premarketing Approvals (PMAs) and PMA supplements – to investigate the “effect of variation in regulatory review time on firms’ choices between radical and incremental innovations.” Daizadeh [3] investigated FDA-registrations demonstrated temporally adjusted structural breaks latent in the data that may be linked to regulation (as cointegration was demonstrated with FDA guidelines data). Daizadeh [5] used the same data to assess the impact of the COVID-19 crisis on the growth of innovation. Daizadeh [6–8] supported the existence of innovative business cycles in medical products using both FDA-registered medicines and medical devices data. To the author’s knowledge, except the works of Onur and Söderberg and Daizadeh, there are no other works that use FDA-registration data to pursue an investigation into the innovation dynamics of medical product development, and specifically an empirical link to the regulatory context.
Regulation as the Number of FDA-Issued Guidelines
Medical product guidelines have received considerable attention by various stakeholders in the literature. Oftentimes, these discussions focus on the impact of one or more guideline(s) on a specific aspect of a process [11, 12] or of the medical product [13]. Infrequently, there is a request for more guidance [14]. A completely missed opportunity, however, is the use of medical product guidelines as a metric in itself – e.g., as a proxy for regulatory complexity (regulation) – a high (low) number of guidelines over a given time-period may indicate periods of a need for high (low) policy interpretation intensive activities. Like the construction of the innovation index, the monthly value of the regulatory index is comprised of the monthly number of FDA-issued guidelines across both medical devices and medicines. Daizadeh [4] provided the first commentary not only suggesting that an assessment of guidelines should be performed as to better understand the effectiveness of such guidelines to the medical product industry but also to resolve any perceived gaps (e.g., when a new guideline would be appreciated or needed).
-
(2)
Temporal evolution of these metrics provide insight into US medical product development.
As it may take decade or longer from ideation to registration of a medical product, flows of multiple registrations may indicate innovation saltuses; ebbs in registration may indicate innovation abysses [4, 9]. Similarly, flows of new guidelines may imply new and complex medical product legislation,ebbs may concordantly imply periods of legislationary stagnation. The requirement for new medical product legislation may be due to accommodating new technologies (e.g., cell and genetic therapiesFootnote 2), further research in a specific target population (e.g., Pediatric Research Equity Act of 2003), or enabling novel processes (e.g., real-world evidenceFootnote 3).
-
(3)
There exists some sort of interplay between the innovation and regulation metrics.
Daizadeh [3] found that medicinal FDA-registrations and FDA-issued guidelines were cointegrated implying a potential causal relationship [15]. Given the complexities of the time series (non-stationarity, non-linearity, multiple structural change points), however, causality assessments were not performed at the time. Cognizant of the difference in time lags (a registration may take a decade or so, while a guideline may take months to years), it would make sense for such integration between these (and the other) time series data at a certain level. The reason for this lies in the purpose of the guideline. If guidelines are meant to facilitate medical product development in a certain area (let’s say orphan medicines), then it may be predicted that there would be an imbalance in the number of registrations in that specific topic area shortly thereafter. Or, if guidelines are meant to facilitate or expedite compliance of some type (e.g., COVID-19 diagnostic kits), they may be promulgated shortly after such a technology was introduced. This cointegration would thus provide the theoretical foundations for interdependency, which would support for a direct view (through, e.g., regression) of regulation and innovation using these proxy metrics, a goal of this work.
This paper is outlined as follows: First, the conceptual model and data collection is outlined. Second, we present the overall approach to analysis. Third, the results of the analysis are presented and interpreted. Lastly, the paper concludes with a summary statement, and a discussion on the limitations of the approach. To aid the reader, allow replication, and potential extension, all data and results are presented in the Supplementary Materials section of this article.
Conceptual Model and Empirical Data
Conceptual Model
A general conceptual framework to describe the evolution of innovation may be framed as follows:
Here, the left-hand side of Eq. 1 reflects represents the time-dependent total innovative capacity of the medical product (MPI) sector comprised of all sponsors which has successfully prosecuted at least one FDA-registered medical product. The right-hand side of Eq. 1 is comprised of states of extrinsic, intrinsic, and stochastic influences. There may exist a state in which there is a co-existing mixture of intrinsic conditional to an extrinsic force. A good example may be a new or updated regulation (extrinsic) that may result in hiring of a certain skill set (intrinsic) to perform a specific task under the compliant process.
This description is intentionally vague as currently there are no economic models detailing a model to fully recapitulate US FDA medical product registrations. Nonetheless, it provides a necessary scaffold to begin the process of detailing such an approach. Regulation, an independent variable of interest to derive the dependent variable is based on the premise of this work.
The key assumption of this model is that MPI would be proportional to a medical product regulatory (MPR) influence of an unknown degree (α), which would be time dependent as both the regulations and the subsequent interpretation and implementation by the firm would be serially correlated (with some unknown time lag); i.e.,
Equation 2 conceptually recognizes that the regulation-innovation relationship is relatively weak relative to other factors influencing the medical product development lifecycle, such as risks to technical success [16], access to financing [17], and so on.
A metric for MPI is assumed to be the sum of FDA-registered medical products, defined as the sum of medicinal (biologics license applications (BLA), new and abbreviate new drug applications (NDA/ANDA), and corresponding supplements) and medical device (premarket approvals and notifications (PMAs/PMNs)) registrations. It is understood that each FDA-registration equates to an innovative success (as market access is formally not allowed if registration cannot be attained) for given medical product (asset, a) in at least one approved indication (i) from a named sponsor(s) (firm, f) at a given timestamp t (which is aggregated over a given month, t’), as follows:
The regulatory influence is defined as the monthly tally of all FDA-issued guidelines, which takes on the form, for a given medical product or process overseeing medical product development:
The question posed is to empirically test the existence of an interdependency between MPI and MPR, and, if existence is confirmed, the order of the proportionality, α.
Data Sources
The method for data-collection, data-cull, and data-formatting have been previously described in the literature [3] through 2022b) and include data from 1976 to 2020 as summarized here:
-
MPI: The Medical Product Innovation metric is constructed as follows:
-
Medical Devices: PMNs and PMAs data obtained from the FDA websites: https://www.fda.gov/medical-devices/510k-clearances/downloadable-510k-files and https://www.fda.gov/medical-devices/device-approvalsdenials-and-clearances/pma-approvals. The data were read into EXCEL, the monthly counts derived.
-
For medicines, the following field ‘All Approvals and Tentative Approvals by Month’ was used on the collecting report from the site: https://www.accessdata.fda.gov/scripts/cder/daf/. The report includes any original or supplemental biologicals (BLA/sBLA), new (NDA/sNDA) or abbreviated (including tentative ANDA/sANDA) application approved by the Center for Drug Evaluation and Research (CDER).
-
-
MPR: The Medical Product Regulatory metric is constructed from data obtained from FDA repository accessible at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents. For medicines, the Product filter was used to collect those from Biologics and Drugs, or FDA Organization for CDER; for medical devices, the FDA Organization for CDRH was used.
Each search produced data that was exported to Excel for integration and duplication removal. The totality of the data was then exported from Excel, via a comma-separated file, and imported into the R Program for data analyses [18]: R version 4.2.1 (2022–06-23 ucrt)).
The data for analysis is comprised of 8 time series. The four components that form the bases of the investigational space, as described above; and four derived time series: two of which are the monthly number of FDA-registered medical products (MPI) and corresponding FDA-issued medical product guidelines (MPR) (each respectively derived from a sum of the underlying time series). Given that it is anticipated that MPI is weakly associated with MPR, two additional time series were derived, which are the cumulative sum of MPI and MPR, to increase single-to-noise ratio. Structurally, this approach is sensical as ongoing maintenance to retain the registrational status; similarly, guidelines continue to be used of reference even though they may be tactical value. It is possible that the registrational status or guideline is rescinded (withdrawn) by FDA (see, e.g., in the case of accelerated approvals for cancer drugsFootnote 4). Assuming such cases are rare, these results are not considered to be affected; additional testing is necessary (see Discussion below).
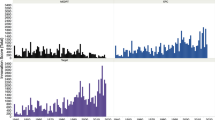
A graphical view of the derived data is depicted in Figs. 1 and 2. Figure 1 (top) presents the time evolution of the number of FDA-registered medical products. Notice the gradual but steady rise into the 1990s following by a decade of stability, until a steep drop is observed at the turn of the century given way to a similar trend into 2020. Whereas Fig. 1 (bottom) shows the slow-to-rise number of FDA-issued guidelines until the mid-2010s, when a rapid rise occurred. A cumulative view of the data as shown in Fig. 2 (top and bottom), however, shows steady momentum throughout the reporting period.
Analyses and Results
The analysis approach is as follows: calculate descriptive and dynamic statistics to inform on methodology for assessing interdependence, which is defined as non-spurious correlation with bi-directional (symmetrical) causality [19, 20]. If evidence of interdependence is elucidated, then proceed to regression analysis to estimate α.
Determine Descriptive and Dynamic Statistics of the Variables in the Study
As the derived data is the focus of this analysis, and since the statistics of the bases data set are described elsewhere, the baseline descriptive and dynamic characteristics statistics of the variables used in this study are presented in Supplementary Materials [21–31]. It is found that the time series characteristics vary in terms of skew, kurtosis, normality, linearity, seasonality, but with similar nonstationary and long-memory (lag) characteristics. There is a significant time lag (on the order of 1–3 years) for both registrations as well as guidelines. These lags suggest latent waves of registrations and guidelines promulgations that may reflect the productivity of industry (innovation, approvals) and/or the FDA (regulation; guidelines) (as shown in Daizadeh [3–9]).
Fortunately, while it is challenging to analyze multiple structural breaks in concomitantly nonstationary and non-linear data, wavelet transform analysis may be used to gain insight into such complex datasets [32–34]. The wavelet power spectra [35] recapitulate and refine the flows latent in the time domain, demonstrating when short and long periodicities appear as a function of time. Reviewing Fig. 3, notice the 8-year and 16-year periodicities appearing from 1990–2020 in the FDA-registered medical products (Fig. 3 Top Left), while the FDA-issued medical product guidelines had a concentration of short-term (≤ 2 year) periodicities appearing over blocks of a 5–10 years (1995–2000, 2005–2010, 2015–2020) (Fig. 3 Top Right). Of particular interest is the consistently long-periodicities (8 and 16 year) spanning the domain from 1980 to 2020 observed in the charts presenting the cumulative data (Fig. 3 bottom). In the same figures, rapid and strong appearance of additional periodicities are observed in the cumulative FDA-issued medical products guidelines but not in the corresponding cumulative FDA-registered medical products. The complex structure recapitulates the complex processes comprising the medical product development process. We use these findings to inform on mapping the regulatory to the innovation parameter (as presented in Fig. 4).
Interdependency: Estimate Correlation and Causality of the Variables in the Study
Given the (primarily) time series non-stationarity, the Spearman method was used to estimate correlation between the time series (Table I) [18]. As these results is new research to the literature, correlation values across all the time series in the data are presented. Interestingly, varying correlation strengths were observed across all pair-wise variables analyzed. From relatively weak (0.18) to strong (~ 1). These values are consistent with the various spectra in the time and frequency domains including the early cointegration studies described, e.g., in Daizadeh [3]. Of particular interest is the strong correlation coefficients for the cumulative variables (FDA-registrations and associated guidelines) indicating strong coupling. From a subgroup analysis, medical device guidelines cross-inform those of medicines (e.g., in the case of combination product development) consistent with Daizadeh [9].
Causality estimation was then performed by the Variable-Lag Transfer Entropy (VLTE) method ([36]; R Package: VLTimeCausality). As mentioned, the multivariate time series intrinsic statistics are complex: normalizing or transforming the data (or even attempting to fit within the common Granger causality assessment scheme) was unrealistic. Thus, a transfer entropy-type calculation was preferred [37, 38], particularly one that could consider the presumed non-fixed (variable) time lag (as discussed above). Table II presents the data across all variables used in this study. While there may be specific interest in certain variables, we focus our attention on the cumulative FDA-issued medical product guidelines and corresponding FDA-registrations, which are casually bi-directionally (symmetric) coupled.
Given the strong correlation and symmetry of the derived cumulative FDA-registered medical products and associated FDA-issued guidelines, a wavelet coherence analysis [35] is performed primarily to better understand the nature of the interaction. As shown in Fig. 3 (bottom) and Fig. 5, long-term (16 year) periodicities are well-illustrated with additional shorter-term (4 year) periodicities arising in the mid-2015s. The arrows, which are lead/lag indicators, appear toward 16 to 32 years (Fig. 5). While it is difficult to ascertain, the arrows seem circular suggesting a bidirectional relationship, recapitulating the causality assessment and strong correlation observed from the non-wavelet approach (that is, Tables I and II).
In summary, interdependency between the metrics, notably the cumulative FDA-registrations and its corresponding cumulative FDA-issued guidelines, are demonstrated as strong correlation and causal assessments are present in the complementary analyses. The question now becomes can this be quantified.
Determining the Relationship between Regulation and Innovation: The Exponent α
To calculate the characteristic of the relationship, α, a simple regression is needed. First, a curvilinear relationship is visually confirmed when the cumulative FDA-issued medical product guidelines is plotted against the cumulative FDA-registered medical products. Three regression analyses are attempted: linear, quadratic, and cubic [39]. Regression correlation coefficients is used to determine the best fit. The following was found: Linear R2 = 0.874, quadratic R2 = 0.964, and cubic R2 = 0.978. The quadratic equation was determined to be the best fit as cubic only gave incremental difference. Thus, α is estimated to be a roughly inverted quadratic scale over the entire time-period analyzed. As presented in Fig. 4, visually, α seems to evolve early in a positive manner, saturating and then inverting, a key finding of this work.
Discussion and Conclusion
US Medical Product Regulation and Innovation Nexus
An understanding of medical product development – including those factors that may accelerate or hinder its innovation – is crucial given the importance of access to quality, safe, and effective medical products to quality of life. In this article, an attempt is made to describe the impact of regulation and innovation within the United States medical product industry using a hypothesis driven approach driven by novel metrics and statistical estimates.
To the author’s knowledge, while the scholarly literature on the general question of regulatory influences in innovation in the US medical product is largeFootnote 5, it is amorphous with certain efforts concentrated: (1) on a certain type of medical product – either medical devices [40] or medicines [41], (2) on the faults of a specific implemented regulation (or guideline or on requests for additional regulatory clarity [42]; or (3) on simply sharing regulatory knowledge to a select audience [43]. Importantly, this is a first research inquiry into the impact of regulation onto innovation that considers a clear statistically testable hypothesis that treats the industry holistically. The approach uses two direct (surrogates) metrics of innovation and regulation directly applicable to medical products and which recapitulates the evolution of the FDA as well as the medical product industry, as it integrates temporal data (from 1976 to 2020).
This work finds that regulation and innovation are:
-
Complex entities (like other econometrics or scientometrics variables): metrics that ebb and flow in time in a non-stationary, non-linear, non-contiguous, and with a long memory manner
-
Interdependent: correlatively and bidirectionally (symmetrically) co-influencing and co-moving variables concomitantly reacting with (likely the same or similar) extrinsic forces
-
Time dependence: Steep linear in construct early in lifecycle but then reaching an inverted (exponentially negatively square or cubic) structure after a period of stability
If the results of this analysis hold after further scrutiny, it suggests that in its early stages regulations supported, if not accelerated, innovation, over time, however, regime change led to the current state in which regulatory complexity may be now hindering innovation.
Study Limitations: Data and Analysis
These results and subsequent interpretations presented here depend on several noteworthy potential shortcomings, as follow:
The data was collected from the publicly accessible FDA website. To the author’s knowledge, there is no public presentation of the processes (e.g., auditing) used to collect the data. The number of and accompanied metadata are large; manual culling was required to isolate the variables of interest to prepare for this analysis. Thus, while every effort was taken to minimally process the data, and while relying on the FDA ‘system of record,’ there is residual uncertainty in the integrity of the final datasets.
From an analysis perspective, it is critical for the reader to understand that the author has constructed a cumulative medical product data record for both registrations and guidelines, for which the key results rest on a statistical approach (viz., determining the statistical characteristics of the data, estimating interdependency and thus regression). The cumulative medical product data assumes that the FDA and sponsor continue their prosecution of the medical product and guideline from its inception onward; that is, the individual metrics accumulate over time. Should the number of guidelines (or registrations) materially decrease, then the ratio of registrations to guidelines would change and such the curve may or may not invert. Confirmation of the database is outstanding and may also comprise corollary investigations. Also, the collection method did not take into number of withdrawn records for either registrations or guidelines. The FDA or the sponsor may have withdrawn / rescinded / retired a registration or guideline. It may be possible (but challenging) to estimate through sensitivity analyses (partly informed by FDA or sponsor media communications) superannuated records, with caveat. For example, a sponsor may withdraw a registration without informing the market (e.g., for patent expired assets with minimal to no commercial value), creating a difficult to ascertain degree of difference between a relative truth and its estimate in the analysis.
Lastly, the causality assessment using VLTE is relatively new and as such additional testing using different econo/sciento-metrics may be appreciated to better understand the algorithm’s limitations. Potentially other algorithms may also be used to cross-check the analysis; however, to the author’s knowledge, none thus far take into regards both the variable lag as well as structural complexities of such data.
Future Directions
The use of FDA metrics data presents a unique opportunity to explore the evolution of the US medical product industry from an economic perspective. Like intellectual property, stock market, and/or other metrics, the waves of change in the time series metrics may be directly associated with environment changes. However, unlike such approaches, they may not be as inter-correlated with other metrics (see, e.g., [44, 45]) or within themselves [46, 47], and thus react relatively early to changes to various micro-, meso-, macro-economic changes, given their relatively long lag-times (on the order of years to decades). Further, the metrics proposed are generally restricted to FDA-regulated industries. Thus, further avenues to consider, beyond reconfirming the results presented:
-
Medical product subsector analysis was not performed for this study; however, it would be straightforward to consider medical devices and medicines separately, as the industry actors are different. Further, the approach may be further extended within sectors to specific types of modalities, pioneers versus generics/biosimilars, delivery modes, and so on.
-
While medicinal lag is well known [48], determining (a median or average) time lags from promulgation of new legislation to guideline production, as a mark of efficiency of the process, is currently unknown [49]. Here a value was given based on intrinsic changes to the time series,however, an objective reference is needed to better understand the effects of when guidances are issued and when the affected industry actors react, as it may add much needed context to the effect of regulation on the day-to-day operations of the firm and potential impact on innovativeness.
-
Beyond medical products, FDA guidance documents are also issued for veterinary products, tobacco, and foods. In principle, therefore, novel metrics may be constructed to consider innovation in these other sectors. Similarly, as other governmental agencies also use guidance documents, opportunities arise to use such regulated documentation to better understand industry response times and/or capabilities to drive new product development.
-
Analytically, predicting rates of medical product development is critical to estimating future work force size, resource needs, and so on (Daizadeh, 2021e). Thus, this work may establish additional input variables to consider when developing such a model; predicting the number of guidance documents may also be of interest given the importance of considering regulatory complexity in medical product design.
There are probably other ideas as well; nonetheless, the goal is to better understand the industrial regulatory economics.
Conclusion
Irrespective of the residual uncertainty in the data and analysis, acknowledging the study limitations and seeking future directions, this work investigates and empirically identifies a potential regulation-innovation nexus in US medical product development through the use of novel metrics that seeks to understand the dynamics of the broad medical product (medical devices and medicines) industry. In summary, considering the elements that may affect innovation such those outlined in this work, we may optimize the rate of discovery-to-delivery for expediting quality, safe, and effective medical products, which is critical to all.
Data Availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Notes
The author adopts the term ‘guideline’ in lieu of ‘guidance’ (as defined under 21 CFR 10.115(b1-b3)) due to ease of reading and alignment to international terminology (see [9] for discourse).
FDA Guidance for Human Somatic Cell Therapy and Gene Therapy. Guidance for Industry. March 1998. Docket Number: FDA-2009-D-0132–0016. Accessible from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-human-somatic-cell-therapy-and-gene-therapy. Accessed on 11-Nov-2022.
Framework for FDA’s Real-World Evidence dated: Dec 2018. Accessible from https://www.fda.gov/media/120060/download. Accessed on 11-Nov-2022.
On 19-Nov-2022, the author found, for example, the following keyword search on scholar.google.com (1) approximately 2000 records using the search string: ‘("regulation" and "innovation") and ("medical product" or "medical device" or "medicine" or "drug")’; (2) 27,000 records with ‘("regulation" and "innovation") and ("medical device")’; (3) 2,070,000 records with ‘("regulation" and "innovation") and ("medicine").’
References
Stewart RB. Regulation, innovation, and administrative law: a conceptual framework. Calif L Rev. 1981;69:1256. https://doi.org/10.15779/Z38JF29
Aghion P, Akcigit U, Howitt P. What do we learn from Schumpeterian growth theory? In Handbook of economic growth 2014;2:515–563. Elsevier. https://doi.org/10.3386/w18824https://www.nber.org/papers/w18824. Accessed 15Apr 2023.
Daizadeh I. Investigating rates of food and drug administration approvals and guidances in drug development: a structural breakpoint/cointegration timeseries analysis. Ther Innov Regul Sci. 2020a;54:1056–67. https://doi.org/10.1007/s43441-020-00123-5.
Daizadeh I. Since the Mid-2010s FDA Drug and Biologic Guidelines have been growing at a faster clip than prior years: is it time to analyze their effectiveness? Ther Innov Regul Sci. 2020b;55:437–9. https://doi.org/10.1007/s43441-020-00233-0.
Daizadeh I. Has the COVID-19 crisis affected the growth of United States Food and Drug Administration Drug Approvals? The answer is not yet! a time series (forecasting) study. Ther Innov Regul Sci. 2020c;55:553–7. https://doi.org/10.1007/s43441-020-00249-6.
Daizadeh, I. US FDA Drug approvals are persistent and polycyclic: insights into economic cycles, innovation dynamics, and national policy. Ther Innov Regul Sci. 2021a;55:743–754. https://doi.org/10.1007/s43441-021-00279-8.
Daizadeh, I. Seasonal and Secular Periodicities Identified in the Dynamics of US FDA Medical Devices (1976–2020): Portends Intrinsic Industrial Transformation and Independence of Certain Crises. Ther Innov Regul Sci 2021b. https://doi.org/10.1007/s43441-021-00334-4 Note: This work was recalled due to a publisher error; readers may also view the preprint version: https://doi.org/10.48550/arXiv.2107.05347.
Daizadeh I. Singular secular Kuznets-like period realized amid industrial transformation in US FDA medical devices: a perspective on growth from 1976 to 2020. Expert Rev Med Devices. 2022a;19(10):745–56. https://doi.org/10.1080/17434440.2022.2139919.
Daizadeh I. Why did the number of US FDA medical device guidelines begin to rise in the mid-2010s? A perspective. Exp Rev Med Devices. 2022b. https://doi.org/10.1080/17434440.2022.2159378.
Onur I, Söderberg M. The impact of regulatory review time on incremental and radical innovation: evidence from the high-risk medical device market. J Regul Econ. 2020;57:134–58. https://doi.org/10.1007/s11149-020-09401-4.
Samaei M, McGregor AJ, Jenkins MR. Inclusion of women in FDA-regulated premarket clinical trials: A call for innovative and recommended action. Contemp Clin Tri. 2022;116(2022):106708. https://doi.org/10.1016/j.cct.2022.106708
Tarver ME, Neuland C. Integrating patient perspectives into medical device regulatory decision making to advance innovation in kidney disease. Clin J Am Soc Nephrol. 2021;16(4):636–638. https://doi.org/10.2215/CJN.11510720
Renukuntla J, Palakurthi SS, Bolla PK, Clark BA, Boddu SHS, Manda P, Sockwell S, Charbe NB, Palakurthi S. Advances in in-vitro bioequivalence testing methods for complex ophthalmic generic products. Int J Pharma. 2022;627:122209. https://doi.org/10.1016/j.ijpharm.2022.122209
Fanse S., Bao Q, Burgess DJ. Long-acting intrauterine systems: Recent advances, current challenges, and future opportunities. Adv Drug Deliver Rev. 2022;191:114581. https://doi.org/10.1016/j.addr.2022.114581
Granger CWJ. Causality, cointegration, and control. J Econ Dyn Control. 1988;12(2–3):551–9. https://doi.org/10.1016/0165-1889(88)90055-3.
van der Graaf PH. Probability of success in drug development. Clin Pharmacol Ther. 2022;111:983–5. https://doi.org/10.1002/cpt.2568.
Lo AW, Thakor RT. Financing biomedical innovation. Annu Rev Financ Econ. 2022;14:231–270. https://doi.org/10.1146/annurev-financial-031721-081537.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2022. URL https://www.R-project.org/. Accessed 15 Apr 2023.
Back WB, Tarver JD. Interdependence of population and economic variables in personal incomes. The Southwestern Social Science Quarterly Vol. 40, Supplement: Annual Meeting Number 1959;22–32. Accessible from https://www.jstor.org/stable/42866324. Accessed 15 Apr 2023.
Pasinetti LL. Causality and interdependence in econometric analysis and in economic theory. Struct Chang Econ Dyn. 2019;49:357–63. https://doi.org/10.1016/j.strueco.2018.09.008.
Revelle W. psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, Illinois, USA; 2022. https://CRAN.R-project.org/package=psychVersion=2.2.9. Accessed 15 Apr 2023.
Gross J, Ligges U. nortest: Tests for Normality. R package version 1.0-4. 2015. https://CRAN.R-project.org/package=nortest. Accessed 15 Apr 2023.
Trapletti A, Hornik K. tseries: Time Series Analysis and Computational Finance. R package version 0.10-52. 2022. https://cran.r-project.org/web/packages/tseries/index.html. Accessed 15 Apr 2023.
Qiu D. aTSA: Alternative Time Series Analysis. R package version 3.1.2. 2015. https://CRAN.R-project.org/package=aTSA. Accessed 15 Apr 2023.
Garcia C. nonlinearTseries: Nonlinear Time Series Analysis. R package version 0.2.12. 2022. https://CRAN.R-project.org/package=nonlinearTseries. Accessed 15 Apr 2023.
Hyndman R, Athanasopoulos G, Bergmeir C, Caceres G, Chhay L, O'Hara-Wild M, Petropoulos F, Razbash S, Wang E, Yasmeen F. forecast: Forecasting functions for time series and linear models. R package version 8.1; 2022. https://pkg.robjhyndman.com/forecast/. Accessed 15 Apr 2023.
Hyndman RJ, Khandakar Y. Automatic time series forecasting: the forecast package for R. J Stat Softw. 2008;26(3):1–22. https://doi.org/10.18637/jss.v027.i03.
Leschinski C. LongMemoryTS: Long Memory Time Series. R package version 0.1.0; 2019. https://CRAN.R-project.org/package=LongMemoryTS. Accessed15 Apr 2023.
Ollech D. seastests: Seasonality Tests. R package version 0.15.4; 2021. https://CRAN.R-project.org/package=seastests. Accessed 15 Apr 2023.
Wei T, Simko V. R package 'corrplot': Visualization of a Correlation Matrix (Version 0.92); 2021. https://github.com/taiyun/corrplot. Accessed 15 Apr 2023.
Harrell Jr F. Hmisc: Harrell Miscellaneous. R package version 4.7-1;2022. https://CRAN.R-project.org/package=Hmisc. Accessed 15 Apr 2023.
Schulte JA. Wavelet analysis for non-stationary, nonlinear time series. Nonlin Processes Geophys. 2016;23:257–67. https://doi.org/10.5194/npg-23-257-2016.
Sang Y-F. A review on the applications of wavelet transform in hydrology time series analysis. Atmos Res.2013;122:8–15. https://doi.org/10.1016/j.atmosres.2012.11.003.
Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteor Soc. 1998;79(1):61–78. https://doi.org/10.1175/1520-0477(1998)079%3c0061:APGTWA%3e2.0.CO;2.
Roesch A, Schmidbauer H. WaveletComp: Computational Wavelet Analysis. R package version 1.1; 2018. https://CRAN.R-project.org/package=WaveletComp. Accessed 15 Apr 2023.
Amornbunchornvej C, Zheleva E, Berger-Wolf T. Variable-lag Granger Causality and Transfer Entropy for Time Series Analysis. ACM Transactions on Knowledge Discovery from Data. 2021;15(4), Article 67.
Mao X, Shang P. Transfer entropy between multivariate time series. Commun Nonlinear Sci Numer Simul. 2017;47:338–47. https://doi.org/10.1016/j.cnsns.2016.12.008.
Dhifaoui Z, Khalfaoui R, Ben-Jabeur S, Abedin MZ. Exploring the effect of climate risk on agricultural and food stock prices fresh evidence from Emd-Based variable-lag transfer entropy analysis. Available at SSRN: https://doi.org/10.2139/ssrn.4163681.
Martins dos Santos W. regr.easy: Easy Linear, Quadratic and Cubic Regression Models. R package version 1.0.1. 2022. https://CRAN.R-project.org/package=regr.easy. Accessed 15 Apr 2023.
Horst A, McDonald F. Uncertain but not unregulated: medical product regulation in the light of three-dimensional printed medical products. 3D Print Addit Manuf. 2020. 248–257. https://doi.org/10.1089/3dp.2020.0076.
Bouchard RA, Sawani J, McLelland C, Sawicka M. The pas de deux of pharmaceutical regulation and innovation: who’s leading whom. Berkeley Tech LJ. 2009;24(4):1461. https://www.jstor.org/stable/24120586. Accessed 15 Apr 2023.
Gutierrez L, Cauchon NS, Christian TR, Giffin MJ, Abernathy MJ. The confluence of innovation in therapeutics and regulation: recent CMC considerations. J Pharm Sci. 2020;109(12):3524–3534. https://doi.org/10.1016/j.xphs.2020.09.025.
Amur S, Frueh FW, Lesko LJ, Huang S-M. Integration and use of biomarkers in drug development, regulation and clinical practice: a US regulatory perspective. Biomark Med. 2008;2(3). https://doi.org/10.2217/17520363.2.3.305.
Daizadeh I. An intellectual property-based corporate strategy: an R&D spend, patent, trademark, media communication, and market price innovation agenda. Scientometrics. 2009;80(3):731–46. https://doi.org/10.1007/s11192-008-2105-9.
Daizadeh I. Issued US patents, patent-related global academic and media publications, and the US market indices are inter-correlated, with varying growth patterns. Scientometrics. 2007;73:29–36. https://doi.org/10.1007/s11192-007-1749-1.
Daizadeh I. Trademark and patent applications are structurally near identical and cointegrated: Implications for studies in innovation. Iberoamerican J Sci Measure Commun. 2021c;1(2). https://doi.org/10.47909/ijsmc.33
Daizadeh I. Leveraging latent persistency in the United States patent and trademark applications to gain insight into the evolution of an innovation-driven economy. 2021d; 1(3). https://doi.org/10.47909/ijsmc.32
Wardell WM. Introduction of new therapeutic drugs in the United States and Great Britain: an international comparison. Clin Pharmacol Ther. 1973;14. https://doi.org/10.1002/cpt1973145773.
Yackee SW. Guidance on regulatory guidance: what the government needs to know and do to engage the public; 2021. Accessible on https://www.businessofgovernment.org/sites/default/files/Guidance%20on%20Regulatory%20Guidance.pdf. Accessed 21 Nov 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author is an employee of Takeda Pharmaceuticals; however, this work was completed independently of his employment. The views expressed in this article may not represent those of his employer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daizadeh, I. The Impact of US Medical Product Regulatory Complexity on Innovation: Preliminary Evidence of Interdependence, Early Acceleration, and Subsequent Inversion. Pharm Res 40, 1541–1552 (2023). https://doi.org/10.1007/s11095-023-03512-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03512-1