Abstract
The present review summarizes the use of differential scanning calorimetry (DSC) and scattering techniques in the context of protein formulation design and characterization. The scattering techniques include wide angle X-ray diffractometry (XRD), small-angle neutron scattering (SANS) and small-angle X-ray scattering (SAXS). While DSC is valuable for understanding thermal behavior of the excipients, XRD provides critical information about physical state of solutes during freezing, annealing and in the final lyophile. However, as these techniques lack the sensitivity to detect biomolecule-related transitions, complementary characterization techniques such as small-angle scattering can provide valuable insights.









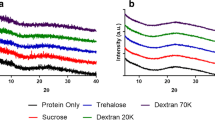
Adapted from Ref. [88] with permission from the Royal Society of Chemistry.

Similar content being viewed by others
Abbreviations
- API:
-
Active Pharmaceutical Ingredient
- ASA:
-
Anti-Streptavidin Antibody
- B 22 :
-
Second virial coefficient
- DSC:
-
Differential Scanning Calorimetry
- k D :
-
Diffusion interaction parameter
- LDH:
-
Lactate dehydrogenase
- mAb:
-
Monoclonal Antibody
- MbCO:
-
Carboxy-myoglobin
- Mdsc:
-
Modulated DSC
- MHH:
-
Mannitol Hemihydrate
- NaP:
-
Sodium phosphate
- NIST:
-
National Institute of Standards and Technology
- NISTmAb:
-
NISTmAb Primary Sample 8670
- P188:
-
Poloxamer 188
- PBS:
-
Phosphate Buffered Saline
- PPI:
-
Protein-Protein Interactions
- PS 20:
-
Polysorbate 20
- SANS:
-
Small-angle Neutron Scattering
- SAS:
-
Small-angle Scattering
- SAXS:
-
Small-angle X-ray Scattering
- SEC:
-
Size Exclusion Chromatography
- SRM:
-
Standard Reference Material
- TBA:
-
tert-Butyl alcohol
- Te:
-
Eutectic temperature
- Tg”, Tg’ and Tg:
-
Glass transition temperature
- tSA:
-
Tetrameric Streptavidin Antibody
- WANS:
-
Wide-angle Neutron Scattering
- WAXS:
-
Wide-angle X-ray Scattering
- XRD:
-
X-ray Diffractometry
References
Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1–3):307–26.
Carpenter JF, Chang BS, Garzon-Rodriguez W, Randolph TW. Rational design of stable lyophilized protein formulations: theory and practice. Rational design of stable protein formulations. 2002 109–133.
Thakral S, Sonje J, Munjal B, Suryanarayanan R. Stabilizers and their interaction with formulation components in frozen and freeze-dried protein formulations. Adv Drug Deliv Rev. 2021;173:1–19.
W W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60.
Sundaramurthi P, Suryanarayanan R. Calorimetry and complementary techniques to characterize frozen and freeze-dried systems. Adv Drug Deliv Rev. 2012;64(5):384–95.
Tang XC, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21(2):191–200.
Bhatnagar BS, Tchessalov S, Lewis LM, Johnson R. Freeze drying of biologics. In. Encyclopedia of Pharmaceutical Science and Technology, Six Volume Set: CRC Press. 2013 p. 1673–1722.
Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–63.
Connolly BD, Le L, Patapoff TW, Cromwell MEM, Moore JMR, Lam P. Protein Aggregation in Frozen Trehalose Formulations: Effects of Composition, Cooling Rate, and Storage Temperature. J Pharm Sci. 2015;104(12):4170–84.
Singh SK, Kolhe P, Mehta AP, Chico SC, Lary AL, Huang M. Frozen state storage instability of a monoclonal antibody: aggregation as a consequence of trehalose crystallization and protein unfolding. Pharm Res. 2011;28(4):873–85.
Gómez G. Crystallization-related pH changes during freezing of sodium phosphate buffer solutions. In.: University of Michigan; 1995.
Pikal-Cleland KA, Rodriguez-Hornedo N, Amidon GL, Carpenter JF. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. Arch Biochem Biophys. 2000;384(2):398–406.
Archer DG. Thermodynamic properties of the NaCl+ H2O system l. Thermodynamic properties of NaCl (cr). J Phys Chem Ref Data. 1992 21(1):1–21.
Kasraian K, DeLuca PP. Thermal analysis of the tertiary butyl alcohol-water system and its implications on freeze-drying. Pharm Res. 1995 12(4):484–490.
Bhatnagar BS, Sonje J, Shalaev E, Martin SW, Teagarden DL, Suryanarayanan R. A refined phase diagram of the tert-butanol–water system and implications on lyophilization process optimization of pharmaceuticals. Phys Chem Chem Phys. 2020;22(3):1583–90.
Shalaev EY, Franks F, Echlin P. Crystalline and Amorphous Phases in the Ternary System Water− Sucrose− Sodium Chloride. J Phys Chem. 1996;100(4):1144–52.
Chatterjee K, Shalaev EY, Suryanarayanan R. Partially crystalline systems in lyophilization: I. Use of ternary state diagrams to determine extent of crystallization of bulking agent. J Pharm Sci. 2005 94(4):798–808.
Thorat AA, Suryanarayanan R. Characterization of phosphate buffered saline (PBS) in frozen State and after Freeze-drying. Pharm Res. 2019;36(7):98.
Nail SL, Jiang S, Chongprasert S, Knopp SA. Fundamentals of freeze-drying. Pharm Biotechnol. 2002;14:281–360.
Nakagawa K, Morishita D, Suzuki T, Sano N. Experimental and computational evaluation of the degree of micro-collapse formations in freeze-dried cakes. Drying Technol. 2022 1–13.
Johnson RE, Kirchhoff CF, Gaud HT. Mannitol-sucrose mixtures–versatile formulations for protein lyophilization. J Pharm Sci. 2002;91(4):914–22.
Searles JA, Carpenter JF, Randolph TW. Annealing to optimize the primary drying rate, reduce freezing-induced drying rate heterogeneity, and determine Tg′ in pharmaceutical lyophilization. J Pharm Sci. 2001;90(7):872–87.
Telang C, Yu L, Suryanarayanan R. Effective inhibition of mannitol crystallization in frozen solutions by sodium chloride. Pharm Res. 2003;20(4):660–7.
Liao X, Krishnamurthy R, Suryanarayanan R. Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol—implications in freeze-drying. Pharm Res. 2005;22(11):1978–85.
Knopp MM, Löbmann K, Elder DP, Rades T, Holm R. Recent advances and potential applications of modulated differential scanning calorimetry (mDSC) in drug development. Eur J Pharm Sci. 2016;87:164–73.
Chang LL, Milton N, Rigsbee D, Mishra DS, Tang XC, Thomas LC, Pikal MJ. Using modulated DSC to investigate the origin of multiple thermal transitions in frozen 10% sucrose solutions. Thermochim Acta. 2006;444(2):141–7.
Pansare SK, Patel SM. Practical considerations for determination of glass transition temperature of a maximally freeze concentrated solution. AAPS PharmSciTech. 2016;17(4):805–19.
Kharatyan T, Gopireddy SR, Ogawa T, Kodama T, Nishimoto N, Osada S, Scherließ R, Urbanetz NA. Quantitative Analysis of Glassy State Relaxation and Ostwald Ripening during Annealing Using Freeze-Drying Microscopy. Pharmaceutics. 2022;14(6):1176.
Surana R, Pyne A, Rani M, Suryanarayanan R. Measurement of enthalpic relaxation by differential scanning calorimetry—effect of experimental conditions. Thermochim Acta. 2005;433(1–2):173–82.
Shamblin SL, Tang X, Chang L, Hancock BC, Pikal MJ. Characterization of the time scales of molecular motion in pharmaceutically important glasses. J Phys Chem B. 1999;103(20):4113–21.
Pyne A, Surana R, Suryanarayanan R. Enthalpic relaxation in frozen aqueous trehalose solutions. Thermochim Acta. 2003;405(2):225–34.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12.
Her L-M, Nail SL. Measurement of glass transition temperatures of freeze-concentrated solutes by differential scanning calorimetry. Pharm Res. 1994;11(1):54–9.
Levine H, Slade L. Thermomechanical properties of small-carbohydrate–water glasses and ‘rubbers’ Kinetically metastable systems at sub-zero temperatures. J Chem Soc, Faraday trans I. 1988;84(8):2619–33.
Sacha GA, Nail SL. Thermal analysis of frozen solutions: multiple glass transitions in amorphous systems. J Pharm Sci. 2009;98(9):3397–405.
Zhu M, Yu L. Polyamorphism of D-mannitol. J Phys Chem. 2017;146(24):244503.
Hancock BC, Zografi G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm Res. 1994;11(4):471–7.
Yoshioka S, Forney KM, Aso Y, Pikal MJ. Effect of sugars on the molecular motion of freeze-dried protein formulations reflected by NMR relaxation times. Pharm Res. 2011;28(12):3237–47.
Breen E, Curley J, Overcashier D, Hsu C, Shire S. Effect of moisture on the stability of a lyophilized humanized monoclonal antibody formulation. Pharm Res. 2001;18(9):1345–53.
Sonje J, Thakral S, Mayhugh B, Sacha G, Nail S, Srinivasan J, Suryanarayanan R. Mannitol Hemihydrate in Lyophilized Protein Formulations: Impact of its Dehydration During Storage on Sucrose Crystallinity and Protein Stability. Int J Pharm. 2022 121974.
Chang LL, Shepherd D, Sun J, Tang XC, Pikal MJ. Effect of sorbitol and residual moisture on the stability of lyophilized antibodies: Implications for the mechanism of protein stabilization in the solid state. J Pharm Sci. 2005;94(7):1445–55.
Izutsu K-i, Yoshioka S, Terao T. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm Res. 1993;10(8):1232–7.
Bhatnagar B, Tchessalov S. Advances in Freeze Drying of Biologics and Future Challenges and Opportunities. Drying Technologies for Biotechnology and Pharmaceutical Applications. 2020 137–177.
Burger A, Henck J-O, Hetz S, Rollinger JM, Weissnicht AA, Stöttner H. Energy/Temperature Diagram and Compression Behavior of the Polymorphs of d-Mannitol. J Pharm Sci. 2000;89(4):457–68.
Kim AI, Akers MJ, Nail SL. The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J Pharm Sci. 1998;87(8):931–5.
Anko M, Bjelošević M, Planinšek O, Trstenjak U, Logar M, Grabnar PA, Brus B. The formation and effect of mannitol hemihydrate on the stability of monoclonal antibody in the lyophilized state. Int J Pharm. 2019;564:106–16.
Srinivasan JM, Wegiel LA, Hardwick LM, Nail SL. The Influence of Mannitol Hemihydrate on the Secondary Drying Dynamics of a Protein Formulation: A Case Study. J Pharm Sci. 2017;106(12):3583–90.
Varshney DB, Kumar S, Shalaev EY, Kang S-W, Gatlin LA, Suryanarayanan R. Solute crystallization in frozen systems–use of synchrotron radiation to improve sensitivity. Pharm Res. 2006;23(10):2368–74.
Kuwamoto S, Akiyama S, Fujisawa T. Radiation damage to a protein solution, detected by synchrotron X-ray small-angle scattering: dose-related considerations and suppression by cryoprotectants. J Synchrotron Radiat. 2004;11(6):462–8.
Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12(5):505–23.
Bhatnagar B, Zakharov B, Fisyuk A, Wen X, Karim F, Lee K, Seryotkin Y, Mogodi M, Fitch A, Boldyreva E. Protein/ice interaction: high-resolution synchrotron X-ray diffraction differentiates pharmaceutical proteins from lysozyme. J Phys Chem B. 2019;123(27):5690–9.
Sundaramurthi P, Suryanarayanan R. Trehalose crystallization during freeze-drying: implications on lyoprotection. J Phys Chem Lett. 2010;1(2):510–4.
Singh SK. Sucrose and trehalose in therapeutic protein formulations. In. Challenges in Protein Product Development: Springer; 2018 p. 63–95.
Cavatur RK, Suryanarayanan R. Characterization of phase transitions during freeze-drying by in situ X-ray powder diffractometry. Pharm Dev Technol. 1998;3(4):579–86.
Thorat AA, Munjal B, Geders TW, Suryanarayanan R. Freezing-induced protein aggregation - Role of pH shift and potential mitigation strategies. J Control Release. 2020;323:591–9.
Kett VL, Fitzpatrick S, Cooper B, Craig DQ. An investigation into the subambient behavior of aqueous mannitol solutions using differential scanning calorimetry, cold stage microscopy, and X-ray diffractometry. J Pharm Sci. 2003;92(9):1919–29.
Mehta M, Bhardwaj SP, Suryanarayanan R. Controlling the physical form of mannitol in freeze-dried systems. Eur J Pharm Biopharm. 2013;85(2):207–13.
Rodrigues MA, Rego P, Geraldes V, Connor LE, Oswald ID, Sztucki M, Shalaev E. Mannitol Crystallization at Sub-Zero Temperatures: Time/Temperature-Resolved Synchrotron X-ray Diffraction Study and the Phase Diagram. J Phys Chem Lett. 2021;12:1453–60.
Hawe A, Frieß W. Impact of freezing procedure and annealing on the physico-chemical properties and the formation of mannitol hydrate in mannitol–sucrose–NaCl formulations. Eur J Pharm Biopharm. 2006;64(3):316–25.
Izutsu K-i, Yosohika S, Terao T. Effect of mannitol crystallinity on the stabilization of enzymes during freeze-drying. Chem Pharm Bull. 1994;42(1):5–8.
Thakral S, Sonje J, Suryanarayanan R. Anomalous behavior of mannitol hemihydrate: Implications on sucrose crystallization in colyophilized systems. Int J Pharm. 2020 119629.
Lu X, Pikal MJ. Freeze-drying of mannitol–trehalose–sodium chloride-based formulations: The impact of annealing on dry layer resistance to mass transfer and cake structure. Pharm Dev Technol. 2004;9(1):85–95.
Dixon D, Tchessalov S, Barry A, Warne N. The impact of protein concentration on mannitol and sodium chloride crystallinity and polymorphism upon lyophilization. J Pharm Sci. 2009;98(9):3419–29.
Larsen HML, Trnka H, Grohganz H. Formation of mannitol hemihydrate in freeze-dried protein formulations—A design of experiment approach. Int J Pharm. 2014;460(1):45–52.
Kulkarni SS, Patel SM, Suryanarayanan R, Rinella JV, Bogner RH. Key factors governing the reconstitution time of high concentration lyophilized protein formulations. Eur J Pharm Biopharm. 2021;165:361–73.
Kulkarni SS, Suryanarayanan R, Rinella JV Jr, Bogner RH. Mechanisms by which crystalline mannitol improves the reconstitution time of high concentration lyophilized protein formulations. Eur J Pharm Biopharm. 2018;131:70–81.
Al-Hussein A, Gieseler H. The effect of mannitol crystallization in mannitol–sucrose systems on LDH stability during freeze-drying. J Pharm Sci. 2012;101(7):2534–44.
Jacques DA, Trewhella J. Small-angle scattering for structural biology—Expanding the frontier while avoiding the pitfalls. Protein Sci. 2010;19(4):642–57.
Rambo RP, Tainer JA. Bridging the solution divide: comprehensive structural analyses of dynamic RNA, DNA, and protein assemblies by small-angle X-ray scattering. Curr Opin Struct Biol. 2010;20(1):128–37.
Schneidman-Duhovny D, Kim SJ, Sali A. Integrative structural modeling with small angle X-ray scattering profiles. BMC Struct Biol. 2012;12(1):1–12.
Rambo RP, Tainer JA. Accurate assessment of mass, models and resolution by small-angle scattering. Nature. 2013;496(7446):477–81.
Gabel F. Small-angle neutron scattering for structural biology of protein–RNA complexes. In. Methods in Enzymology: Elsevier. 2015 p. 391–415.
Jeffries CM, Graewert MA, Blanchet CE, Langley DB, Whitten AE, Svergun DI. Preparing monodisperse macromolecular samples for successful biological small-angle X-ray and neutron-scattering experiments. Nat Protoc. 2016;11(11):2122–53.
Trewhella J. Small-angle scattering and 3D structure interpretation. Curr Opin Struct Biol. 2016;40:1–7.
Tuukkanen AT, Spilotros A, Svergun DI. Progress in small-angle scattering from biological solutions at high-brilliance synchrotrons. IUCrJ. 2017;4(5):518–28.
Mahieu E, Gabel F. Biological small-angle neutron scattering: recent results and development. Acta Crystallographica Section D: Structural Biology. 2018;74(8):715–26.
Brosey CA, Tainer JA. Evolving SAXS versatility: solution X-ray scattering for macromolecular architecture, functional landscapes, and integrative structural biology. Curr Opin Struct Biol. 2019;58:197–213.
Da Vela S, Svergun DI. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. Current research in structural biology. 2020;2:164–70.
Trewhella J. Recent advances in small-angle scattering and its expanding impact in structural biology. Structure. 2021
Whitten AE, Cai S, Trewhella J. MULCh: modules for the analysis of small-angle neutron contrast variation data from biomolecular assemblies. J Appl Crystallogr. 2008;41(1):222–6.
Sarachan KL, Curtis JE, Krueger S. Small-angle scattering contrast calculator for protein and nucleic acid complexes in solution. J Appl Crystallogr. 2013;46(6):1889–93.
Krueger S. Small-angle neutron scattering contrast variation studies of biological complexes: Challenges and triumphs. Curr Opin Struct Biol. 2022;74:102375.
Krueger S. Designing and Performing Biological Solution Small-Angle Neutron Scattering Contrast Variation Experiments on Multi-component Assemblies. Adv Exp Med Biol. 2017;1009:65–85.
Zaccai NR, Sandlin CW, Hoopes JT, Curtis JE, Fleming PJ, Fleming KG, Krueger S. Deuterium labeling together with contrast variation small-angle neutron scattering suggests how Skp captures and releases unfolded outer membrane proteins. In. Methods in Enzymology: Elsevier. 2016 p. 159–210.
Dong Y-D, Boyd BJ. Applications of X-ray scattering in pharmaceutical science. Int J Pharm. 2011;417(1–2):101–11.
Boyd BJ, Rades T. Applications of small angle X-ray scattering in pharmaceutical science. In. Analytical Techniques in the Pharmaceutical Sciences: Springer; 2016 p. 339–360.
Curtis JE, Nanda H, Khodadadi S, Cicerone M, Lee HJ, McAuley A, Krueger S. Small-angle neutron scattering study of protein crowding in liquid and solid phases: lysozyme in aqueous solution, frozen solution, and carbohydrate powders. J Phys Chem B. 2012;116(32):9653–67.
Curtis JE, McAuley A, Nanda H, Krueger S. Protein structure and interactions in the solid state studied by small-angle neutron scattering. Faraday Discuss. 2012;158(1):285–99.
Stradner A, Sedgwick H, Cardinaux F, Poon WC, Egelhaaf SU, Schurtenberger P. Equilibrium cluster formation in concentrated protein solutions and colloids. Nature. 2004;432(7016):492–5.
Cardinaux F, Stradner A, Schurtenberger P, Sciortino F, Zaccarelli E. Modeling equilibrium clusters in lysozyme solutions. EPL (Europhysics Letters). 2007;77(4):48004.
Shukla A, Mylonas E, Di Cola E, Finet S, Timmins P, Narayanan T, Svergun DI. Absence of equilibrium cluster phase in concentrated lysozyme solutions. PNAS. 2008;105(13):5075–80.
Porcar L, Falus P, Chen W-R, Faraone A, Fratini E, Hong K, Baglioni P, Liu Y. Formation of the Dynamic Clusters in Concentrated Lysozyme Protein Solutions. J Phys Chem Lett. 2010;1(1):126–9.
Bergman MJ, Garting T, Schurtenberger P, Stradner A. Experimental Evidence for a Cluster Glass Transition in Concentrated Lysozyme Solutions. J Phys Chem B. 2019;123(10):2432–8.
Olsson C, Swenson J. The role of disaccharides for protein–protein interactions–a SANS study. Mol Phys. 2019;117(22):3408–16.
Yearley EJ, Zarraga IE, Shire SJ, Scherer TM, Gokarn Y, Wagner NJ, Liu Y. Small-angle neutron scattering characterization of monoclonal antibody conformations and interactions at high concentrations. Biophys J. 2013;105(3):720–31.
Yearley EJ, Godfrin PD, Perevozchikova T, Zhang H, Falus P, Porcar L, Nagao M, Curtis JE, Gawande P, Taing R. Observation of small cluster formation in concentrated monoclonal antibody solutions and its implications to solution viscosity. Biophys J. 2014;106(8):1763–70.
Godfrin PD, Zarraga IE, Zarzar J, Porcar L, Falus P, Wagner NJ, Liu Y. Effect of hierarchical cluster formation on the viscosity of concentrated monoclonal antibody formulations studied by neutron scattering. J Phys Chem B. 2016;120(2):278–91.
Castellanos MM, Pathak JA, Leach W, Bishop SM, Colby RH. Explaining the non-Newtonian character of aggregating monoclonal antibody solutions using small-angle neutron scattering. Biophys J. 2014;107(2):469–76.
Castellanos MM, Clark NJ, Watson MC, Krueger S, McAuley A, Curtis JE. Role of molecular flexibility and colloidal descriptions of proteins in crowded environments from small-angle scattering. J Phys Chem B. 2016;120(49):12511–8.
Castellanos MM, Snyder JA, Lee M, Chakravarthy S, Clark NJ, McAuley A, Curtis JE. Characterization of monoclonal antibody–protein antigen complexes using small-angle scattering and molecular modeling. Antibodies. 2017;6(4):25.
Xu AY, Clark NJ, Pollastrini J, Espinoza M, Kim H-J, Kanapuram S, Kerwin B, Treuheit MJ, Krueger S, McAuley A. Effects of Monovalent Salt on Protein-Protein Interactions of Dilute and Concentrated Monoclonal Antibody Formulations. Antibodies. 2022;11(2):24.
Schiel JE, Mouchahoir CA. Glycan Analysis of NIST mAb Reference Material. 2015.
Castellanos MM, Howell SC, Gallagher DT, Curtis JE. Characterization of the NISTmAb reference material using small-angle scattering and molecular simulation. Anal Bioanal Chem. 2018;410(8):2141–59.
Castellanos MM, Mattison K, Krueger S, Curtis JE. Characterization of the NISTmAb Reference Material using small-angle scattering and molecular simulation part II: concentrated solutions. Anal Bioanal Chem. 2018;410(8):2161–71.
Xu AY, Castellanos MM, Mattison K, Krueger S, Curtis JE. Studying excipient modulated physical stability and viscosity of monoclonal antibody formulations using small-angle scattering. Mol Pharm. 2019;16(10):4319–38.
Chou SG, Soper AK, Khodadadi S, Curtis JE, Krueger S, Cicerone MT, Fitch AN, Shalaev EY. Pronounced microheterogeneity in a sorbitol–water mixture observed through variable temperature neutron scattering. J Phys Chem B. 2012;116(15):4439–47.
Yuan X, Krueger S, Sztucki M, Jones RL, Curtis JE, Shalaev E. Phase Behavior of Poloxamer 188 Aqueous Solutions at Subzero Temperatures: A Neutron and X-ray Scattering Study. J Phys Chem B. 2021.
Khodadadi S, Clark NJ, McAuley A, Cristiglio V, Curtis JE, Shalaev EY, Krueger S. Influence of sorbitol on protein crowding in solution and freeze-concentrated phases. Soft Matter. 2014;10(23):4056–60.
Yuan X, Krueger S, Shalaev E. Protein-surfactant and protein-protein interactions during freeze and thaw: a small-angle neutron scattering study of lysozyme solutions with polysorbate and poloxamer. J Pharm Sci. 2022.
Sonje J, Thakral S, Krueger S, Suryanarayanan R. Reversible Self-Association in Lactate Dehydrogenase during Freeze-Thaw in Buffered Solutions Using Neutron Scattering. Mol Pharm. 2021;18(12):4459–74.
Jaenicke R. Reassociation and reactivation of lactic dehydrogenase from the unfolded subunits. Eur J Pharm Biochem. 1974;46(1):149–55.
Giuffrida S, Panzica M, Giordano F, Longo A. SAXS study on myoglobin embedded in amorphous saccharide matrices. Eur Phys J E. 2011;34(9):1–7.
Longo A, Giuffrida S, Cottone G, Cordone L. Myoglobin embedded in saccharide amorphous matrices: water-dependent domains evidenced by small angle X-ray scattering. Phys Chem Chem Phys. 2010;12(25):6852–8.
Giuffrida S, Cottone G, Bellavia G, Cordone L. Proteins in amorphous saccharide matrices: Structural and dynamical insights on bioprotection. Eur Phys J E. 2013;36(7):1–12.
Phan-Xuan T, Bogdanova E, Millqvist Fureby A, Fransson J, Terry AE, Kocherbitov V. Hydration-induced structural changes in the solid state of protein: A SAXS/WAXS study on lysozyme. Mol Pharm. 2020;17(9):3246–58.
Cristiglio V, Sztucki M, Wu C, Shalaev E. Impact of lyoprotectors on protein-protein separation in the solid state: Neutron-and X-ray-scattering investigation. Biochimica et Biophysica Acta (BBA)-General Subjects. 2022 1866(5):130101.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflicts of interests/competing interests in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sonje, J., Thakral, S., Krueger, S. et al. Enabling Efficient Design of Biological Formulations Through Advanced Characterization. Pharm Res 40, 1459–1477 (2023). https://doi.org/10.1007/s11095-023-03495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03495-z