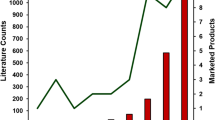
Abstract
Purpose
Plasticizers are commonly used in the preparation of amorphous solid dispersions (ASDs) with the main goal of aiding processability; however, to the best of our knowledge, the impact of plasticizers on drug release has not been explored. The goal of this study was to evaluate diverse plasticizers, including glycerol and citrate derivatives, as additives to increase the drug loading where good drug release could be achieved from copovidone (PVPVA)-based dispersions, focusing on high glass transition (Tg) drugs, atazanavir (ATZ) and ledipasvir (LED).
Methods
ASDs were prepared using the high Tg compounds, atazanavir (ATZ) and ledipasvir (LED), as model drugs. Release was evaluated using surface normalized dissolution testing. Differential scanning calorimetry was used to measure glass transition temperature and water vapor sorption was performed on select samples.
Results
The presence of a plasticizer at 5% w/w for ATZ and 10% w/w for LED ASDs, led to improved drug release. For ATZ ASDs, in the absence of plasticizer, release was very poor at drug loadings of 10% w/w and above. Good release was obtained for plasticized ASDs up to a drug loading of 25%. The corresponding improvement for LED was from 5 to 20% DL. Interestingly, for a low Tg compound, ritonavir, relatively smaller improvements in release as a function of drug loading were achieved through plasticizer incorporation.
Conclusions
The use of plasticizers represents a potential new strategy to increase drug loading in ASDs for high Tg compounds with a low tendency to crystallize and may help improve a major limitation of ASD formulations, namely the high excipient burden.
Graphical Abstract












Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- API:
-
Active Pharmaceutical Ingredient
- ASD:
-
Amorphous Solid Dispersion
- ATZ:
-
Atazanavir
- DL:
-
Drug Loading
- DSC:
-
Differential Scanning Calorimetry
- DVS:
-
Dynamic vapor sorption (DVS)
- GTA:
-
Glycerol triacetate
- GTB:
-
Glycerol tributyrate
- LED:
-
Ledipasvir
- LLPS:
-
Liquid-Liquid phase separation
- LoC:
-
Limit of Congruency
- PVPVA:
-
Polyvinylpyrrolidone/vinyl acetate or copovidone
- PXRD:
-
Powder X-ray diffraction
- RTV:
-
Ritonavir
- T g :
-
Glass transition temperature
- TBAC:
-
Tributyl o-acetylcitrate
- TBC:
-
Tri-n-butyl citrate
- TEAC:
-
Triethyl o-acetylcitrate
- TEC:
-
Triethyl citrate
- TFA:
-
Trifluoroacetic Acid
References
Williams HD, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65:315–499.
Jermain SV, Brough C, Williams RO. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery – an update. Int J Pharm. 2018;535:379–92.
Van Den Mooter G. The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol. 2012;9:e79–85.
Solanki NG, Gumaste SG, Shah AV, Serajuddin ATM. Effects of surfactants on itraconazole-hydroxypropyl methylcellulose acetate succinate solid dispersion prepared by hot melt extrusion. II: Rheological analysis and extrudability testing. J Pharm Sci. 2019;108:3063–3073.
Schittny A, Philipp-Bauer S, Detampel P, Huwyler J, Puchkov M. Mechanistic insights into effect of surfactants on oral bioavailability of amorphous solid dispersions. J Control Release. 2020;320:214–25.
He Y, Ho C. Amorphous solid dispersions: utilization and challenges in drug discovery and development. J Pharm Sci. 2015;104:3237–58.
Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231:131–44.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–75.
Taylor LS, Zhang GGZ. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016;101:122–42.
Corrigan OI. Mechanisms of dissolution of fast release solid dispersions. Drug Dev Ind Pharm. 1985;11:697–724.
Van Duong T, Van den Mooter G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part II: amorphous carriers. Exp Opin Drug Deliv. 2016;13:1681–1694.
Indulkar AS, Lou X, Zhang GGZ, Taylor LS. Insights into the dissolution mechanism of ritonavir-copovidone amorphous solid dispersions: importance of congruent release for enhanced performance. Mol Pharm. 2019;16:1327–39.
Lang B, Liu S, McGinity JW, Williams RO. Effect of hydrophilic additives on the dissolution and pharmacokinetic properties of itraconazole-enteric polymer hot-melt extruded amorphous solid dispersions. Drug Dev Ind Pharm. 2016;42:429–45.
Saboo S, Mugheirbi NA, Zemlyanov DY, Kestur US, Taylor LS. Congruent release of drug and polymer: a “sweet spot” in the dissolution of amorphous solid dispersions. J Control Release. 2019;298:68–82.
Saboo S, Moseson DE, Kestur US, Taylor LS. Patterns of drug release as a function of drug loading from amorphous solid dispersions: a comparison of five different polymers. Eur J Pharm Sci. 2020;155: 105514.
Que C, et al. Insights into the dissolution behavior of ledipasvir-copovidone amorphous solid dispersions: role of drug loading and intermolecular interactions. Mol Pharm. 2019;16:5054–67.
Saboo S, Kestur US, Flaherty DP, Taylor LS. Congruent release of drug and polymer from amorphous solid dispersions: insights into the role of drug-polymer hydrogen bonding, surface crystallization, and glass transition. Mol Pharm. 2020;17:1261–75.
Yang R, et al. Drug release and nanodroplet formation from amorphous solid dispersions: insight into the roles of drug physicochemical properties and polymer selection. Mol Pharm. 2021. https://doi.org/10.1021/acs.molpharmaceut.1c00055.
Kumar V, et al. Binary and ternary solid dispersions of an anticancer preclinical lead, IIIM-290: in vitro and in vivo studies. Int J Pharm. 2019;570: 118683.
Prasad D, Chauhan H, Atef E. Role of molecular interactions for synergistic precipitation inhibition of poorly soluble drug in supersaturated drug-polymer-polymer ternary solution. Mol Pharm. 2016;13:756–65.
Davis MT, Potter CB, Mohammadpour M, Albadarin AB, Walker GM. Design of spray dried ternary solid dispersions comprising itraconazole, soluplus and HPMCP: Effect of constituent compositions. Int J Pharm. 2017;519:365–72.
Prasad D, Chauhan H, Atef E. Amorphous stabilization and dissolution enhancement of amorphous ternary solid dispersions: Combination of polymers showing drug-polymer interaction for synergistic effects. J Pharm Sci. 2014;103:3511–23.
Song Y, et al. Acid-base interactions of polystyrene sulfonic acid in amorphous solid dispersions using a combined UV/FTIR/XPS/ssNMR study. Mol Pharm. 2016;13:483–92.
Nie H, et al. Investigating the interaction pattern and structural elements of a drug-polymer complex at the molecular level. Mol Pharm. 2015. https://doi.org/10.1021/acs.molpharmaceut.5b00162.
Song Y, et al. Investigation of drug-excipient interactions in lapatinib amorphous solid dispersions using solid-State NMR spectroscopy. Mol Pharm. 2015;12:857–66.
Singh S, et al. Supersolubilization and amorphization of a model basic drug, haloperidol, by interaction with weak acids. Pharm Res. 2013;30:1561–73.
Meng F, Ferreira R, Zhang F. Effect of surfactant level on properties of celecoxib amorphous solid dispersions. J Drug Deliv Sci Technol. 2019;49:301–7.
Alhayali A, Tavellin S, Velaga S. Dissolution and precipitation behavior of ternary solid dispersions of ezetimibe in biorelevant media. Drug Dev Ind Pharm. 2017;43:79–88.
Janssens S, et al. Formulation and characterization of ternary solid dispersions made up of Itraconazole and two excipients, TPGS 1000 and PVPVA 64, that were selected based on a supersaturation screening study. Eur J Pharm Biopharm. 2008;69:158–66.
Wan LSC, Heng PWS, Chia CGH. Plasticizers and their effects on microencapsulation process by spray-drying in an aqueous system. J Microencapsul. 1992;9:53–62.
Wan LSC, Heng PWS, Chia CGH. Citric acid as a plasticizer for spray-dried microcapsules. J Microencapsul. 1993;10:11–23.
Wan LSC, Heng PWS, Chia CGH. Preparation of coated particles using a spray drying process with an aqueous system_. Int J Pharm. 1991;77:183–91.
Mendonsa N, et al. Manufacturing strategies to develop amorphous solid dispersions : an overview. J Drug Deliv Sci Technol. 2020;55: 101459.
Solanki NG, et al. Effects of surfactants on itraconazole-HPMCAS solid dispersion prepared by hot melt extrusion. I: miscibility and drug release. J Pharm Sci. 2018;1–13. https://doi.org/10.1016/j.xphs.2018.10.058.
Zhu Y, Shah NH, Malick AW, Infeld MH, McGinity JW. Solid-state plasticization of an acrylic polymer with chlorpheniramine maleate and triethyl citrate. Int J Pharm. 2002;241:301–10.
Wu C, McGinity JW. Influence of ibuprofen as a solid-state plasticizer in Eudragit RS 30 D on the physicochemical properties of coated beads. AAPS PharmSciTech. 2001;2(4):24. https://doi.org/10.1208/pt020424.
Correa-Soto CE, Gao Y, Indulkar AS, Zhang GGZ, Taylor LS. Role of surfactants in improving release from higher drug loading amorphous solid dispersions. Int J Pharm. 2022;625: 122120.
Miller DA, DiNunzio JC, Yang W, McGinity JW, Williams RO. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic-to-neutral pH transition. Drug Dev Ind Pharm. 2008;34:890–902.
Immergut EH, Mark HF. Principles of plasticization. In: Plasticization and plasticizers processes. American Chemical Society; 1965. p. 1–26. https://doi.org/10.1021/ba-1965-0048.ch001.
Labrecque LV, Kumar RA, Davé V, Gross RA, Mccarthy SP. Citrate esters as plasticizers for poly(lactic acid). J Appl Polym Sci. 1997;66:1507–13.
Indulkar AS, Box KJ, Taylor R, Ruiz R, Taylor LS. pH-dependent liquid-liquid phase separation of highly supersaturated solutions of weakly basic drugs. Mol Pharm. 2015;12:2365–77.
Ilevbare GA, Taylor LS. Liquid-liquid phase separation in highly supersaturated aqueous solutions of poorly water-soluble drugs: Implications for solubility enhancing formulations. Cryst Growth Des. 2013. https://doi.org/10.1021/cg301679h.
Feldman, D. Applied polymer science, 2nd ed., R. W. Tess and G. W. Poehlein Eds., ACS Symposium Series 285, Washington D.C., 1985, 1341 pp., price: $59.95 U.S. & Canada, $71.95 export price. J Polym Sci C Polym Lett. 1986;24:613–4. https://doi.org/10.1002/pol.1986.140241109.
Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer-surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–29.
Correa Soto CE, et al. Impact of surfactants on the performance of clopidogrel-copovidone amorphous solid dispersions: increased drug loading and stabilization of nanodroplets. Pharm Res. 2022;39:167–188.
Mosquera-Giraldo LI, Trasi NS, Taylor LS. Impact of surfactants on the crystal growth of amorphous celecoxib. Int J Pharm. 2014;461:251–7.
Chen J, Ormes JD, Higgins JD, Taylor LS. Impact of surfactants on the crystallization of aqueous suspensions of celecoxib amorphous solid dispersion spray dried particles. Mol Pharm. 2015;12:533–41.
Indulkar AS, Gao Y, Raina SA, Zhang GGZ, Taylor LS. Impact of monomeric versus micellar surfactant and surfactant-polymer interactions on nucleation-induction times of atazanavir from supersaturated solutions. Cryst Growth Des. 2020;20:62–72.
Yu J, et al. Surface enrichment of surfactants in amorphous drugs: an x-ray photoelectron spectroscopy study. Mol Pharm. 2022;19:654–60.
Saboo S, Bapat P, Moseson DE, Kestur US, Taylor LS. Exploring the role of surfactants in enhancing drug release from amorphous solid dispersions at higher drug loadings. Pharmaceutics. 2021;13:1–22.
Devotta I, Badiger MV, Rajamohanan PR, Ganapathy S. Unusual retardation and enhancement in the polymer dissolution: role of disengagement dynamics. Chem Eng Sci. 1995;50:2557–2569.
Zi P, et al. Solubility and bioavailability enhancement study of lopinavir solid dispersion matrixed with a polymeric surfactant - Soluplus. Eur J Pharm Sci. 2019;134:233–45.
Marcilla A, Beltrán M. Mechanisms of plasticizers action. Handb Plast Third Ed. 2017;119–134. https://doi.org/10.1016/B978-1-895198-97-3.50007-X.
Williams ML, Landel RF, Ferry JD. The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J Am Chem Soc. 1955;77:3701–7.
Cooper WJ, Krasicky PD, Rodriguez F. Dissolution rates of poly(methyl methacrylate) films in mixed solvents. J Appl Polym Sci. 1986;31:65–73.
Cooper WJ, Krasicky PD, Rodriguez F. Effects of molecular weight and plasticization on dissolution rates of thin polymer films. Polymer (Guildf). 1985;26:1069–72.
Devotta I, Mashelkar RA. Role of thermodynamic and kinetic factors in polymer dissolution in mixed solvents. Chem Eng Commun. 1996;156:31–43.
Devotta I, Ambeskar VD, Mandhare AB, Mashelkar RA. The life time of a dissolving polymeric particle. Chem Eng Sci. 1994;49:645–54.
Ouano AC, Carothers JA. Dissolution dynamics of some polymers: solvent-polymer boundaries. Polym Eng Sci. 1980;20:160–6.
Ouano AC. Dissolution Kinetics of Polymers: Effect of Residual Solvent Content. Organic Coatings and Plastics Chemistry: Preprints of Papers presented at the Meeting of the Ameri vol. 45. Pergamon Press Inc.;1981.
Acknowledgements
The authors would like to thank AbbVie Inc., for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
AbbVie and Purdue University jointly participated in study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. C.E.C.S. and L.S.T. have no additional conflicts of interest to report. A.S.I., Y.G., and G.G.Z.Z. are employees of AbbVie and may own AbbVie stock.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Correa-Soto, C.E., Gao, Y., Indulkar, A.S. et al. Release Enhancement by Plasticizer Inclusion for Amorphous Solid Dispersions Containing High Tg Drugs. Pharm Res 40, 777–790 (2023). https://doi.org/10.1007/s11095-023-03483-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03483-3