Abstract
Purpose
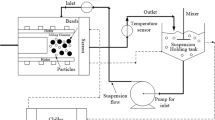
Nanosuspensions have been used for enhancing the bioavailability of poorly soluble drugs. This study explores the temperature evolution during their preparation in a wet stirred media mill using a coupled experimental–enthalpy balance approach.
Methods
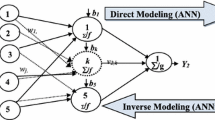
Milling was performed at three levels of stirrer speed, bead loading, and bead sizes. Temperatures were recorded over time, then simulated using an enthalpy balance model by fitting the fraction of power converted to heat ξ. Moreover, initial and final power, ξ, and temperature profiles at 5 different test runs were predicted by power-law (PL) and machine learning (ML) approaches.
Results
Heat generation was higher at the higher stirrer speed and bead loading/size, which was explained by the higher power consumption. Despite its simplicity with a single fitting parameter ξ, the enthalpy balance model fitted the temperature evolution well with root mean squared error (RMSE) of 0.40–2.34°C. PL and ML approaches provided decent predictions of the temperature profiles in the test runs, with RMSE of 0.93–4.17 and 1.00–2.17°C, respectively.
Conclusions
We established the impact of milling parameters on heat generation–power and demonstrated the simulation–prediction capability of an enthalpy balance model when coupled to the PL–ML approaches.
Graphical abstract








Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this manuscript [and its supplementary information file].
Abbreviations
- A :
-
Heat transfer area, m2
- A 1, A 2 :
-
Constants used in the overall heat transfer coefficient calculations, −
- c :
-
Bead volume fraction (loading) in the milling chamber, −
- C p :
-
Specific heat capacity, J/g°C
- D :
-
Diameter, m
- F :
-
Volumetric flow rate of the recirculating suspension, ml/min
- h :
-
Heat transfer coefficient, W/m2°C
- k :
-
Thermal conductivity, W/m°C
- KNN:
-
k-nearest neighborhood
- m :
-
Mass flow rate, g/s
- M :
-
Mass, g
- MAE:
-
Mean absolute error
- ML:
-
Machine learning
- MSE:
-
Mean squared error
- n :
-
Shape factor in thermal conductivity equation, −
- N :
-
Stirrer speed, 1/s
- P :
-
Power applied by the mill stirrer (rotor), W
- PL:
-
Power law
- Pr:
-
Prandtl number, −
- Q :
-
Heat removal rate, W
- R :
-
Radius, m
- Re:
-
Reynolds number, −
- RMSE:
-
Root mean squared error
- t :
-
Milling time, min
- T :
-
Temperature, °C
- U :
-
Overall heat transfer coefficient, W/m2°C
- WSMM:
-
Wet stirred media milling
- YSZ:
-
Yttrium-stabilized zirconia
- μ :
-
Apparent shear viscosity of the suspension, Pa·s
- ξ :
-
Fraction of power converted to heat, −
- ρ :
-
Density, kg/m3
- ω :
-
Stirrer (rotational) speed, rpm
- b:
-
Bead
- B:
-
Product batch side
- ch:
-
Chiller, chiller liquid
- f:
-
Final
- ht.:
-
Holding tank
- j:
-
Jacket side
- L:
-
Equivalent liquid (milled drug suspension)
- lm:
-
Logarithmic mean
- m:
-
Mill chamber
- mix:
-
Mixture of beads and drug suspension
- s:
-
Suspension
- st:
-
Stirrer
- 0:
-
Initial
References
Bergström CA, Charman WN, Porter CJ. Computational prediction of formulation strategies for beyond-rule-of-5 compounds. Adv Drug Deliv Rev. 2016;101:6–21.
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, Porter CJ. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499.
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Li M, Azad M, Davé R, Bilgili E. Nanomilling of drugs for bioavailability enhancement: a holistic formulation-process perspective. Pharmaceutics. 2016;8(2):17.
Salem HF. Sustained-release progesterone nanosuspension following intramuscular injection in ovariectomized rats. Int J Nanomedicine. 2010;5:943.
Van’t Klooster G, Hoeben E, Borghys H, Looszova A, Bouche M-P, van Velsen F, Baert L. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54(5):2042–50.
Bhakay A, Rahman M, Dave RN, Bilgili E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation–Processing aspects and challenges. Pharmaceutics. 2018;10(3):86.
Jenning V, Lippacher A, Gohla S. Medium scale production of solid lipid nanoparticles (SLN) by high pressure homogenization. J Microencapsul. 2002;19(1):1–10.
Beck C, Dalvi SV, Dave RN. Controlled liquid antisolvent precipitation using a rapid mixing device. Chem Eng Sci. 2010;65(21):5669–75.
Peltonen L. Design space and QbD approach for production of drug nanocrystals by wet media milling techniques. Pharmaceutics. 2018;10(3):104.
Singare DS, Marella S, Gowthamrajan K, Kulkarni GT, Vooturi R, Rao PS. Optimization of formulation and process variable of nanosuspension: an industrial perspective. Int J Pharm. 2010;402(1–2):213–20.
Singh SK, Srinivasan K, Gowthamarajan K, Singare DS, Prakash D, Gaikwad NB. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur J Pharm Biopharm. 2011;78(3):441–6.
Patel PJ, Gajera BY, Dave RH. A quality-by-design study to develop Nifedipine nanosuspension: examining the relative impact of formulation variables, wet media milling process parameters and excipient variability on drug product quality attributes. Drug Dev Ind Pharm. 2018;44(12):1942–52.
Ahuja BK, Jena SK, Paidi SK, Bagri S, Suresh S. Formulation, optimization and in vitro–in vivo evaluation of febuxostat nanosuspension. Int J Pharm. 2015;478(2):540–52.
Lehocký R, Pěček D, Štěpánek F. Scale-up from batch to flow-through wet milling process for injectable depot formulation. Eur J Pharm Sci. 2016;95:122–9.
Medarević D, Djuriš J, Ibrić S, Mitrić M, Kachrimanis K. Optimization of formulation and process parameters for the production of carvedilol nanosuspension by wet media milling. Int J Pharm. 2018;540(1):150–61.
Afolabi A, Akinlabi O, Bilgili E. Impact of process parameters on the breakage kinetics of poorly water-soluble drugs during wet stirred media milling: A microhydrodynamic view. Eur J Pharm Sci. 2014;51:75–86.
Parker N, Rahman M, Bilgili E. Impact of media material and process parameters on breakage kinetics–energy consumption during wet media milling of drugs. Eur J Pharm Biopharm. 2020;153:52–67.
Jog R, Burgess DJ. Comprehensive quality by design approach for stable nanocrystalline drug products. Int J Pharm. 2019;564:426–60.
Descamps M, Willart J. Perspectives on the amorphisation/milling relationship in pharmaceutical materials. Adv Drug Deliv Rev. 2016;100:51–66.
Meng T, Qiao F, Ma S, Gao T, Li L, Hou Y, Yang J. Exploring the influence factors and improvement strategies of drug polymorphic transformation combined kinetic and thermodynamic perspectives during the formation of nanosuspensions. Drug Dev Ind Pharm. 2021;47(12):1867–80.
Knieke C, Azad M, Davé R, Bilgili E. A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves. Chem Eng Res Des. 2013;91(7):1245–58.
Bitterlich A, Laabs C, Krautstrunk I, Dengler M, Juhnke M, Grandeury A, Bunjes H, Kwade A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur J Pharm Biopharm. 2015;92:171–9.
Verma S, Kumar S, Gokhale R, Burgess DJ. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406(1–2):145–52.
Aleandri S, Schönenberger M, Niederquell A, Kuentz M. Temperature-induced surface effects on drug Nanosuspensions. Pharm Res. 2018;35(3):69.
NISSO. NISSO HPC | Nippon Soda Co., Ltd. Nissoexcipients.com. Available from: https://www.nissoexcipients.com/hpc-e/care_stable. Accessed 12 May 2022
Eskin D, Zhupanska O, Hamey R, Moudgil B, Scarlett B. Microhydrodynamics of stirred media milling. Powder Technol. 2005;156(2–3):95–102.
Wylie JJ, Koch DL, Ladd AJ. Rheology of suspensions with high particle inertia and moderate fluid inertia. J Fluid Mech. 2003;480:95–118.
Guner G, Kannan M, Berrios M, Bilgili E. Use of bead mixtures as a novel process optimization approach to Nanomilling of drug suspensions. Pharm Res. 2021;38(7):1279–96.
Li M, Alvarez P, Bilgili E. A microhydrodynamic rationale for selection of bead size in preparation of drug nanosuspensions via wet stirred media milling. Int J Pharm. 2017;524(1):178–92.
Toziopoulou F, Malamatari M, Nikolakakis I, Kachrimanis K. Production of aprepitant nanocrystals by wet media milling and subsequent solidification. Int J Pharm. 2017;533(2):324–34.
Garcia F, Le Bolay N, Frances C. Changes of surface and volume properties of calcite during a batch wet grinding process. Chem Eng J. 2002;85(2–3):177–87.
Bilgili E, Guner G. Mechanistic modeling of wet stirred media milling for production of drug Nanosuspensions. AAPS PharmSciTech. 2020;22(1):2.
Azad M, Guner G, Afolabi A, Davé R, Bilgili E. Impact of solvents during wet stirred media milling of cross-linked biopolymer suspensions. Adv Powder Technol. 2021;32(12):4562–75.
Guner G, Yilmaz D, Bilgili E. Kinetic and microhydrodynamic modeling of Fenofibrate Nanosuspension production in a wet stirred media mill. Pharmaceutics. 2021;13(7):1055.
Guner G, Yilmaz D, Eskin D, Bilgili E. Effects of bead packing limit concentration on microhydrodynamics-based prediction of breakage kinetics in wet stirred media milling. Powder Technol. 2022;403:117433.
Li M, Yaragudi N, Afolabi A, Dave R, Bilgili E. Sub-100nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem Eng Sci. 2015;130:207–20.
Annapragada A, Adjei A. Numerical simulation of milling processes as an aid to process design. Int J Pharm. 1996;136(1–2):1–11.
Frances C. On modelling of submicronic wet milling processes in bead mills. Powder Technol. 2004;143:253–63.
Green, Don W., and Marylee Z. Southard, Eds. 2019. Perry's Chemical Engineers' Handbook. 9th ed. New York: McGraw-Hill Education. https://www.accessengineeringlibrary.com/content/book/9780071834087.
Watterson S, Hudson S, Svärd M, Rasmuson ÅC. Thermodynamics of fenofibrate and solubility in pure organic solvents. Fluid Phase Equilib. 2014;367:143–50.
Joshi H, Wilson T. Calorimetric studies of dissolution of hydroxypropyl methylcellulose E5 (HPMC E5) in water. J Pharm Sci. 1993;82(10):1033–8.
Tojo T, Atake T, Mori T, Yamamura H. Heat capacity and thermodynamic functions of zirconia and yttria-stabilized zirconia. J Chem Thermodyn. 1999;31(7):831–45.
Coleman T, Branch MA, Grace A. Optimization toolbox. For use with MATLAB User’s guide for MATLAB. 1999;5. Available from: https://www.dpipe.tsukuba.ac.jp/~naito/optim_tb.pdf. Accessed 12 May 2022.
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Scikit-learn DV. Machine learning in Python. The. J Mach Learn Res. 2011;12:2825–30.
Li M, Alvarez P, Orbe P, Bilgili E. Multi-faceted characterization of wet-milled griseofulvin nanosuspensions for elucidation of aggregation state and stabilization mechanisms. AAPS PharmSciTech. 2018;19(4):1789–801.
Liu G. Application of the Two-Fluid model with kinetic theory of granular flow in liquid–solid fluidized beds. In: Kyzas G, Mitropoulos AC, Eds. Chapter 2, granularity in materials science [Internet]. London: IntechOpen; 2018 [cited 2022 Jul 20]. Available from: https://doi.org/10.5772/intechopen.79696
Cerdeira AM, Gander B, Mazzotti M. Role of milling parameters and particle stabilization on nanogrinding of drug substances of similar mechanical properties. Chemical Engineering & Technology. 2011;34(9):1427–38.
Fogel’Son R, Likhachev E. Temperature dependence of viscosity. Tech Phys. 2001;46(8):1056–9.
Mannheim V. Empirical and scale-up modeling in stirred ball mills. Chem Eng Res Des. 2011;89(4):405–9.
Geankoplis CJ, Hersel AA, Lepek DH. Transport processes and separation process principles: prentice hall. Boston: MA, USA; 2018.
Zhao H, Yu F, Bennett TD, Wadley HNG. Morphology and thermal conductivity of yttria-stabilized zirconia coatings. Acta Mater. 2006;54(19):5195–207.
Peet M, Hasan H, Bhadeshia H. Prediction of thermal conductivity of steel. Int J Heat Mass Transf. 2011;54(11–12):2602–8.
Khan A, Ali HM, Nazir R, Ali R, Munir A, Ahmad B, Ahmad Z. Experimental investigation of enhanced heat transfer of a car radiator using ZnO nanoparticles in H2O–ethylene glycol mixture. J Therm Anal Calorim. 2019;138(5):3007–21.
Gillies RG, Shook CA. Modelling high concentration settling slurry flows. Can J Chem Eng. 2000;78(4):709–16.
Schlichting K, Padture N, Klemens P. Thermal conductivity of dense and porous yttria-stabilized zirconia. J Mater Sci. 2001;36(12):3003–10.
Ramires ML, Nieto de Castro CA, Nagasaka Y, Nagashima A, Assael MJ, Wakeham WA. Standard reference data for the thermal conductivity of water. J Phys Chem Ref Data. 1995;24(3):1377–81.
Bohne D, Fischer S, Obermeier E. Thermal, conductivity, density, viscosity, and Prandtl-numbers of ethylene glycol-water mixtures. Ber Bunsenges Phys Chem. 1984;88(8):739–42.
Acknowledgements
The authors thank Dr. Sayantan Chattoraj of GSK for his invaluable comments on the first draft of this manuscript. The first and last authors thank Nisso America Inc. for donating HPC.
Author information
Authors and Affiliations
Contributions
Gulenay Guner: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Writing - Original Draft, Visualization. Sherif Elashri: Investigation. Mirsad Mehaj: Investigation. Natasha Seetharaman: Investigation. Helen F. Yao: Conceptualization. Donald J. Clancy: Conceptualization, Project administration. Ecevit Bilgili: Conceptualization, Methodology, Formal Analysis, Writing - Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of Interest
This study was funded by GlaxoSmithKline (GSK) through the Research & Development Service Agreement with NJIT entitled “Advanced Modeling of Pharmaceutical Wet Stirred Media Milling Process for the Production of Drug Nanosuspensions” [NJIT Grant Code G2718B0].
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 493 kb)
Appendices
Appendix A
The integration of a differential enthalpy balance for the coolant (chiller liquid) passing through the jacket [51] of the milling chamber leads to following expression for the heat removal rate from the milling chamber and overall enthalpy balance for the chiller liquid:
In the derivation of the above expressions, one assumes plug flow of the chiller liquid inside the jacket [51] and makes a pseudo steady-state approximation due to time-dependence of the variables along with the well-mixedness assumption for the suspension in the mill and the holding tank (spatial invariance of Ts,m and Ts.ht). The chiller liquid temperature entering the milling chamber Tch,in was measured and recorded for each sampling time. Rearrangement of Eq. 15 yields
This equation can be simplified by defining number of transfer units (NTU) as follows:
which is identical to Eq. 7 in the main text. Continuing derivation as:
The r.h.s. of Eq. 15 could be rewritten with NTU as follows:
By inserting Eq. 19 and NTU definition into Eq. 20, Eq. 6 was obtained. Eq. 8 was obtained in a similar fashion.
Appendix B
Overall heat transfer coefficient U for the mill chamber and holding tank was calculated from [51]:
where hB is the heat transfer coefficient of the liquid (product batch) inside the mill chamber or the holding tank, hj is the heat transfer coefficient of the jacket side chiller liquid, R and A are the radius and surface area of the respective chambers, where i and o indices stand for inside and outside. Alm is the logarithmic mean of inside and outside areas. kwall is the thermal conductivity of the wall, which is zirconia for mill chamber with 2.5 W/m°C [52] and stainless steel for holding tank with 15 W/m°C [53]. When U is written in the form as in Eq. 21, the surface area in UA is taken as Ai. hB was calculated using:
in which k is the thermal conductivity of the liquid, A2 is a constant that depends on agitator type, which was taken as 0.54 for mill chamber (disk agitator) and 0.36 for holding tank (paddle agitator). D is the diameter of the chamber, Re is the Reynolds number, and Pr is the Prandtl number. N is the stirrer speed (1/s), μ is viscosity and ρ is density. For the mill chamber, k, μ and ρ were found for the bead–suspension mixture as follows [54,55]:
where kb and ks are thermal conductivities of the beads and the suspension, respectively. kb is 1.8 W/m°C [56] and ks is assumed to be equal to that of water, i.e., 0.607 W/m°C [57]. n was taken as 3 for spherical beads [54]. c is the bead loading. μL and ρL are the viscosity and density of the drug pre-suspension, which were measured as 198 mPa.s and 1030 kg/m3. For hj, the following correlation [51] was used:
Here, A1 and b are constants that are recommended to be 0.0265 and 0.3, respectively, for a cooling system (jacket). v is the jacket liquid velocity. ρj, μj, and Prj were taken as 1.13 g/cm3, 1.448 mPa.s, and 15 respectively, for the glycol–water mixture [58] used in our chiller.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guner, G., Elashri, S., Mehaj, M. et al. An Enthalpy-Balance Model for Timewise Evolution of Temperature during Wet Stirred Media Milling of Drug Suspensions. Pharm Res 39, 2065–2082 (2022). https://doi.org/10.1007/s11095-022-03346-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03346-3