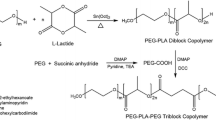
Abstract
Purpose
This study aims to investigate the effect of poly(D, L-lactic acid)10K (PDLLA10K) incorporation on the drug loading and stability of poly(ethylene glycol)2K-block-poly(D, L-lactide)2.4K (mPEG2k-b-PDLLA2.4k) micelles. In addition, a suitable lyophilization protector was screened for this micelle to obtain favorable lyophilized products.
Methods
The incorporation ratios of PDLLA10k were screened based on the particle size and drug loading. The dynamic stability, core viscosity, drug release, stability in albumin, and in vivo pharmacokinetic characteristics of PDLLA10k incorporated micelles were compared with the original micelles. In addition, the particle size variation was used as an indicator to screen the most suitable lyophilization protectant for the micelles. DSC, FTIR, XRD were used to illustrate the mechanism of the lyophilized protectants.
Results
After the incorporation of 5 wt% PDLLA10K, the maximum loading of mPEG2k-b-PDLLA2.4k micelles for TM-2 was increased from 26 wt% to 32 wt%, and the in vivo half-life was increased by 2.25-fold. Various stability of micelles was improved. Also, the micelles with hydroxypropyl-β-cyclodextrin (HP-β-CD) as lyophilization protectants had minimal variation in particle size.
Conclusions
PDLLA10k incorporation can be employed as a strategy to increase the stability of mPEG2k-b-PDLLA2.4k micelles, which can be attributed to the viscosity building effect. HP-β-CD can be used as an effective lyophilization protectant since mPEG and HP-β-CD form the pseudopolyrotaxanesque inclusion complexes.







Similar content being viewed by others
References
Sofias AM, Dunne M, Storm G, Allen C. The battle of "nano" paclitaxel. Adv Drug Deliv Rev. 2017;122:20–30. https://doi.org/10.1016/j.addr.2017.02.003.
Tommasi S, Mangia A, Lacalamita R, Bellizzi A, Fedele V, Chiriatti A, Thomssen C, Kendzierski N, Latorre A, Lorusso V, Schittulli F, Zito F, Kavallaris M, Paradiso A. Cytoskeleton and paclitaxel sensitivity in breast cancer: the role of beta-tubulins. Int J Cancer. 2007;120(10):2078–85. https://doi.org/10.1002/ijc.22557.
Men L, Zhao Y, Lin H, Yang M, Liu H, Tang X, Yu Z. Characterization of in vitro metabolites of TM-2, a potential antitumor drug, in rat, dog and human liver microsomes using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2014;28(20):2162–70. https://doi.org/10.1002/rcm.7003.
Men L, Zhao Y, Lin H, Yang M, Liu H, Shao Y, Fan R, Tang X, Yu Z. Application of an LC-MS/MS method to the pharmacokinetics of TM-2, a potential antitumour agent, in rats. Drug Testing Anal. 2015;7(6):544–9. https://doi.org/10.1002/dta.1711.
Chen YD, Huang ZY. Differences between the quality aspects of various generic and branded docetaxel formulations. Curr Med Res Opin. 2021. https://doi.org/10.1080/03007995.2021.1929895.
Osorno LL, Brandley AN, Maldonado DE, Yiantsos A, Mosley RJ, Byrne ME. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials. 2021;11(2):278. https://doi.org/10.3390/nano11020278.
Zheng X, Xie JZ, Zhang X, Sun WT, Zhao HY, Li YT, Wang C. An overview of polymeric nanomicelles in clinical trials and on the market. Chin Chem Lett. 2021;32(1):243–57. https://doi.org/10.1016/j.cclet.2020.11.029.
Zhang Y, Ren TY, Gou JX, Zhang L, Tao XG, Tian B, Tian P, Yu D, Song J, Liu X, Chao Y, Xiao W, Tang X. Strategies for improving the payload of small molecular drugs in polymeric micelles. J Control Release. 2017;261:352–66. https://doi.org/10.1016/j.jconrel.2017.01.047.
Letchford K, Burt HM. Copolymer micelles and Nanospheres with different in vitro stability demonstrate similar paclitaxel pharmacokinetics. Mol Pharm. 2012;9(2):248–60. https://doi.org/10.1021/mp2002939.
Sun X, Wang G, Zhang H, Hu S, Liu X, Tang J, Shen Y. The blood clearance kinetics and pathway of polymeric micelles in Cancer drug delivery. ACS Nano. 2018;12(6):6179–92. https://doi.org/10.1021/acsnano.8b02830.
Shi L, Zhang J, Zhao M, Tang S, Cheng X, Zhang W, Li W, Liu X, Peng H, Wang Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 2021;13(24):10748–64. https://doi.org/10.1039/d1nr02065j.
Ke X, Ng VWL, Ono RJ, Chan JMW, Krishnamurthy S, Wang Y, et al. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J Control Release. 2014;193:9–26. https://doi.org/10.1016/j.jconrel.2014.06.061.
Wei X, Liao JH, Davoudi Z, Zheng H, Chen JR, Li D, et al. Folate receptor-targeted and GSH-responsive carboxymethyl chitosan nanoparticles containing covalently entrapped 6-mercaptopurine for enhanced intracellular drug delivery in leukemia. Mar Drugs. 2018;16(11):439. https://doi.org/10.3390/md16110439.
Zhang Y, Liu Y, Wang N, Liu H, Gou J, He H, Zhang Y, Yin T, Wang Y, Tang X. Preparation of mPEG-b-PLA/TM-2 micelle lyophilized products by mixed Lyoprotectors and antitumor effect in vivo. AAPS PharmSciTech. 2021;22(1):38. https://doi.org/10.1208/s12249-020-01885-9.
Gou J, Feng S, Xu H, Fang G, Chao Y, Zhang Y, Xu H, Tang X. Decreased Core crystallinity facilitated drug loading in polymeric micelles without affecting their biological performances. Biomacromolecules. 2015;16(9):2920–9. https://doi.org/10.1021/acs.biomac.5b00826.
Allijn IE, Schiffelers RM, Storm G. Comparison of pharmaceutical nanoformulations for curcumin: enhancement of aqueous solubility and carrier retention. Int J Pharm. 2016;506(1–2):407–13. https://doi.org/10.1016/j.ijpharm.2016.04.070.
Vakil R, Kwon GS. Poly(ethylene glycol)-b-poly(epsilon-caprolactone) and PEG-phospholipid form stable mixed micelles in aqueous media. Langmuir. 2006;22(23):9723–9. https://doi.org/10.1021/la061408y.
Kang N, Perron M-È, Prud'homme RE, Zhang Y, Gaucher G, Leroux J-C. Stereocomplex block copolymer micelles: core−shell nanostructures with enhanced stability. Nano Lett. 2005;5(2):315–9. https://doi.org/10.1021/nl048037v.
Wang Q, Liu Y, Pu CG, Zhang HJ, Tan XY, Gou JX, He H, Yin T, Zhang Y, Wang Y, Tang X. Drug-polymer interaction, pharmacokinetics and antitumor effect of PEG-PLA/taxane derivative TM-2 micelles for intravenous drug delivery. Pharm Res. 2018;35(11):13. https://doi.org/10.1007/s11095-018-2477-3.
Miller T, van Colen G, Sander B, Golas MM, Uezguen S, Weigandt M, Goepferich A. Drug loading of polymeric micelles. Pharm Res. 2013;30(2):584–95. https://doi.org/10.1007/s11095-012-0903-5.
Zhuo XZ, Lei T, Miao LL, Chu W, Li XW, Luo LF, Gou J, Zhang Y, Yin T, He H, Tang X. Disulfiram-loaded mixed nanoparticles with high drug-loading and plasma stability by reducing the core crystallinity for intravenous delivery. J Colloid Interface Sci. 2018;529:34–43. https://doi.org/10.1016/j.jcis.2018.05.057.
Yang B, Jiang J, Jiang L, Zheng P, Wang F, Zhou Y, et al. Chitosan mediated solid lipid nanoparticles for enhanced liver delivery of zedoary turmeric oil in vivo. Int J Biol Macromol. 2020;149:108–15. https://doi.org/10.1016/j.ijbiomac.2020.01.222.
Shi CJ, Sun YJ, Wu HY, Zhu CG, Wei GG, Li JF, Chan T, Ouyang D, Mao S. Exploring the effect of hydrophilic and hydrophobic structure of grafted polymeric micelles on drug loading. Int J Pharm. 2016;512(1):282–91. https://doi.org/10.1016/j.ijpharm.2016.08.054.
Yuan Y, Choi K, Choi SO, Kim J. Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system. RSC Adv. 2018;8(35):19791–803. https://doi.org/10.1039/c8ra01467a.
Guo XD, Qian Y, Zhang CY, Nie SY, Zhang LJ. Can drug molecules diffuse into the core of micelles? Soft Matter. 2012;8(39):9989–95. https://doi.org/10.1039/c2sm26200b.
Li Y, Yang L. Driving forces for drug loading in drug carriers. J Microencapsul. 2015;32(3):255–72. https://doi.org/10.3109/02652048.2015.1010459.
Carstens MG, de Jong PHJLF, van Nostrum CF, Kemmink J, Verrijk R, de Leede LGJ, et al. The effect of core composition in biodegradable oligomeric micelles as taxane formulations. Eur J Pharm Biopharm. 2008;68(3):596–606. https://doi.org/10.1016/j.ejpb.2007.08.014.
Xiang HZ, Wang YL, Yang WH, Hu CB, Mu YH, Li JF. Study of the mechanical and thermal properties of poly(lactic acid) and poly(ethylene glycol) block copolymer with molecular dynamics. Int J Polym Anal Charact. 2010;15(4):235–44. https://doi.org/10.1080/10236661003746405.
Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv Drug Deliv Rev. 2002;54(2):191–202. https://doi.org/10.1016/s0169-409x(02)00016-9.
Zhang XJ, Yang QS, Liu X, Shang JJ, Leng JS. Atomistic investigation of the shape-memory effect of amorphous poly(L-lactide) with different molecular weights. Smart Mater Struct. 2020;29(1):11. https://doi.org/10.1088/1361-665X/ab471c.
Hillgren A, Evertsson H, Alden M. Interaction between lactate dehydrogenase and tween 80 in aqueous solution. Pharm Res. 2002;19(4):504–10. https://doi.org/10.1023/a:1015156031381.
Dey A, Banik R, Ghosh S. Temperature comparative studies on self-assembly of sodium dodecyl Sulphate and Didodecyl dimethyl ammonium bromide in aqueous, brine, and Trifluoroethanol media. J Surfactant Deterg. 2021;24(3):459–72. https://doi.org/10.1002/jsde.12385.
Jelonek K, Li SM, Wu XH, Kasperczyk J, Marcinkowski A. Self-assembled filomicelles prepared from polylactide/poly(ethylene glycol) block copolymers for anticancer drug delivery. Int J Pharm. 2015;485(1–2):357–64. https://doi.org/10.1016/j.ijpharm.2015.03.032.
Owen SC, Chan DPY, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7(1):53–65. https://doi.org/10.1016/j.nantod.2012.01.002.
Nicolai T, Colombani O, Chassenieux C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter. 2010;6(14):3111–8. https://doi.org/10.1039/b925666k.
Kelley EG, Murphy RP, Seppala JE, Smart TP, Hann SD, Sullivan MO, Epps TH. Size evolution of highly amphiphilic macromolecular solution assemblies via a distinct bimodal pathway. Nat Commun. 2014;5:10. https://doi.org/10.1038/ncomms4599.
Won Y-Y, Davis HT, Bates FS. Molecular exchange in PEO−PB micelles in water. Macromolecules. 2003;36(3):953–5. https://doi.org/10.1021/ma021439+.
Schantz AB, Saboe PO, Sines IT, Lee HY, Bishop KJM, Maranas JK, Butler PD, Kumar M. PEE-PEO block copolymer exchange rate between mixed micelles is detergent and temperature activated. Macromolecules. 2017;50(6):2484–94. https://doi.org/10.1021/acs.macromol.6b01973.
Myhre S, Amann M, Willner L, Knudsen KD, Lund R. How detergents dissolve polymeric micelles: kinetic pathways of hybrid micelle formation in SDS and block copolymer mixtures. Langmuir. 2020;36(43):12887–99. https://doi.org/10.1021/acs.langmuir.0c02123.
Wen S-N, Chu C-H, Wang Y-C, Huang H-Y, Wang Y-J, Lin J-Y, Lu HT, Wang SJ, Yang CS. Polymer-stabilized micelles reduce the drug rapid clearance in vivo. J Nanomater. 2018;2018:1–7. https://doi.org/10.1155/2018/5818592.
Ma Y-C, Wang J-X, Tao W, Qian H-S, Yang X-Z. Polyphosphoester-based nanoparticles with viscous flow core enhanced therapeutic efficacy by improved intracellular drug release. ACS Appl Mater Interfaces. 2014;6(18):16174–81. https://doi.org/10.1021/am5042466.
Rajdev P, Ghosh S. Fluorescence resonance energy transfer (FRET): a powerful tool for probing amphiphilic polymer aggregates and supramolecular polymers. J Phys Chem B. 2019;123(2):327–42. https://doi.org/10.1021/acs.jpcb.8b09441.
Chen H, Kim S, He W, Wang H, Low PS, Park K, Cheng JX. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo Forster resonance energy transfer imaging. Langmuir. 2008;24(10):5213–7. https://doi.org/10.1021/la703570m.
Liu B, Thayumanavan S. Importance of evaluating dynamic encapsulation stability of amphiphilic assemblies in serum. Biomacromolecules. 2017;18(12):4163–70. https://doi.org/10.1021/acs.biomac.7b01220.
Diezi TA, Bae Y, Kwon GS. Enhanced stability of PEG-block-poly(N-hexyl stearate l-aspartamide) micelles in the presence of serum proteins. Mol Pharm. 2010;7(4):1355–60. https://doi.org/10.1021/mp100069p.
Zhao Y, Fay F, Hak S, Manuel Perez-Aguilar J, Sanchez-Gaytan BL, Goode B, Duivenvoorden R, de Lange Davies C, Bjørkøy A, Weinstein H, Fayad ZA, Pérez-Medina C, Mulder WJM. Augmenting drug–carrier compatibility improves tumour nanotherapy efficacy. Nat Commun. 2016;7(1):11221. https://doi.org/10.1038/ncomms11221.
Koji K, Yoshinaga N, Mochida Y, Hong T, Miyazaki T, Kataoka K, et al. Bundling of mRNA strands inside polyion complexes improves mRNA delivery efficiency in vitro and in vivo. Biomaterials. 2020;261:120332. https://doi.org/10.1016/j.biomaterials.2020.120332.
Mochida Y, Cabral H, Miura Y, Albertini F, Fukushima S, Osada K, Nishiyama N, Kataoka K. Bundled assembly of helical nanostructures in polymeric micelles loaded with platinum drugs enhancing therapeutic efficiency against pancreatic tumor. ACS Nano. 2014;8(7):6724–38. https://doi.org/10.1021/nn500498t.
Makino A, Hara E, Hara I, Yamahara R, Kurihara K, Ozeki E, Yamamoto F, Kimura S. Control of in vivo blood clearance time of polymeric micelle by stereochemistry of amphiphilic polydepsipeptides. J Control Release. 2012;161(3):821–5. https://doi.org/10.1016/j.jconrel.2012.05.006.
Zhao Y, van Rooy I, Hak S, Fay F, Tang J, Davies CDL, et al. Near-infrared fluorescence energy transfer imaging of nanoparticle accumulation and dissociation kinetics in tumor-bearing mice. ACS Nano. 2013;7(11):10362–70. https://doi.org/10.1021/nn404782p.
Zou P, Chen H, Paholak HJ, Sun D. Noninvasive fluorescence resonance energy transfer imaging of in vivo premature drug release from polymeric nanoparticles. Mol Pharm. 2013;10(11):4185–94. https://doi.org/10.1021/mp4002393.
Lin W, Kim D. pH-sensitive micelles with cross-linked cores formed from polyaspartamide derivatives for drug delivery. Langmuir. 2011;27(19):12090–7. https://doi.org/10.1021/la200120p.
Van Domeselaar GH, Kwon GS, Andrew LC, Wishart DS. Application of solid phase peptide synthesis to engineering PEO–peptide block copolymers for drug delivery. Colloids Surf B: Biointerfaces. 2003;30(4):323–34. https://doi.org/10.1016/S0927-7765(03)00125-5.
Ojha T, Hu QZ, Colombo C, Wit J, van Geijn M, van Steenbergen MJ, et al. Lyophilization stabilizes clinical-stage core-crosslinked polymeric micelles to overcome cold chain supply challenges. Biotechnol J. 2021;16(6):e2000212. https://doi.org/10.1002/biot.202000212.
Di Tommaso C, Como C, Gurny R, Moeller M. Investigations on the lyophilisation of MPEG-hexPLA micelle based pharmaceutical formulations. Eur J Pharm Sci. 2010;40(1):38–47. https://doi.org/10.1016/j.ejps.2010.02.006.
Yang ZL, Li XR, Yang KW, Liu Y. Freeze-drying and in vitro release kinetics of poly(ethylene glycol)poly(dl-lactide) block copolymer micelles. Acta Chim Sin. 2007;65(19):2169–74.
ACKNOWLEDGMENTS AND DISCLOSURES
None.
Funding
This work was supported by National Mega-project for Innovative Drugs [No.2019ZX09721001], Liaoning Revitalization Talents Program [XLYC1908031], National Science and Technology Major Project of the Ministry of Science and Technology of China [No.2018ZX09735005], and the Project of Liaoning Provincial Department of Education [2019LQN07], PhD Research Startup Foundation of Liaoning Province [2020BS-128]. National Key R&D Program of China (No. 2020YFE0201700).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 456 kb)
Rights and permissions
About this article
Cite this article
Guo, C., Zhang, Y., Yuan, H. et al. Improved Core Viscosity Achieved by PDLLA10kCo-Incorporation Promoted Drug Loading and Stability of mPEG2k-b-PDLLA2.4k Micelles. Pharm Res 39, 369–379 (2022). https://doi.org/10.1007/s11095-022-03174-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03174-5