Abstract
Purpose
Fexofenadine is a well-identified in vivo probe substrate of P-glycoprotein (P-gp) and/or organic anion transporting polypeptide (OATP). This work aimed to investigate the transplacental pharmacokinetics of fexofenadine enantiomers with and without the selective P-gp inhibitor fluoxetine.
Methods
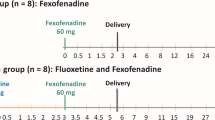
The chiral transplacental pharmacokinetics of fexofenadine-fluoxetine interaction was determined using the ex vivo human placenta perfusion model (n = 4). In the Control period, racemic fexofenadine (75 ng of each enantiomer/ml) was added in the maternal circuit. In the Interaction period, racemic fluoxetine (50 ng of each enantiomer/mL) and racemic fexofenadine (75 ng of each enantiomer/mL) were added to the maternal circulation. In both periods, maternal and fetal perfusate samples were taken over 90 min.
Results
The (S)-(−)- and (R)-(+)-fexofenadine fetal-to-maternal ratio values in Control and Interaction periods were similar (~0.18). The placental transfer rates were similar between (S)-(−)- and (R)-(+)-fexofenadine in both Control (0.0024 vs 0.0019 min−1) and Interaction (0.0019 vs 0.0021 min−1) periods. In both Control and Interaction periods, the enantiomeric fexofenadine ratios [R-(+)/S-(−)] were approximately 1.
Conclusions
Our study showed a low extent, slow rate of non-enantioselective placental transfer of fexofenadine enantiomers, indicating a limited fetal fexofenadine exposure mediated by placental P-gp and/or OATP2B1. The fluoxetine interaction did not affect the non-enantioselective transplacental transfer of fexofenadine. The ex vivo placental perfusion model accurately predicts in vivo placental transfer of fexofenadine enantiomers with remarkably similar values (~0.17), and thus estimates the limited fetal exposure.



Similar content being viewed by others
Data Availability
The data sets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OATP:
-
Organic anion transporting polypeptide
- P-gp:
-
P-glycoprotein
References
Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutics drugs. Clin Pharm Ther. 2011;90:67–76.
Etwel F, Hutson JR, Madadi P, Gareri J, Koren G. Fetal and perinatal exposure to drugs and chemicals: novel biomarkers of risk. Annu Rev Pharmacol Toxicol. 2014;54:295–315.
De Sousa MM, Hirt D, Vinot C, Valade E, Lui G, Pressiat C. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81:646–57.
Myllynen P, Immonen E, Kummu M, Vähäkangas K. Developmental expression of drug metabolizing enzymes and transporter proteins in human placenta and fetal tissues. Expert Opin Drug Metab Toxicol. 2009;5:1483–99.
Staud F, Cerveny L, Ceckova M. Pharmacotherapy in pregnancy; effect of ABC and SLC transporters on drug transport across the placenta and fetal drug exposure. J Drug Target. 2012;20:736–63.
Ellfolk M, Tornio A, Niemi M, Leinonen MK, Lahesmaa-Korpinen AM, Malm H. Placental transporter-mediated drug interactions and offspring congenital anomalies. Br J Clin Pharmacol. 2020;86:868–79.
Daud ANA, Bergman JEH, Oktora MP, Kerstjens-Frederikse WS, Groen H, Bos JH, et al. Maternal use of drug substrates of placental transporters and the effect of transporter-mediated drug interactions on the risk of congenital anomalies. PLoS One. 2017;12:e0173530.
Daud ANA, Bergman JEH, Bakker MK, Wang H, Kerstjens-Frederikse WS, de Walle HEK, et al. P-glycoprotein-mediated drug interactions in pregnancy and changes in the risk of congenital anomalies: a case-reference study. Drug Saf. 2015;38:651–9.
Etwel F, Faught LH, Rieder MJ, Koren G. The risk of adverse pregnancy outcome after first trimester exposure to h1 antihistamines: a systematic review and meta-analysis. Drug Saf. 2017;40:121–32.
Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193:771–7.
Li Q, Mitchell AA, Werler MM, Yau WP, Hernandez-Dıaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract. 2013;1:666–74.
Allegra label FDA (2021). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/20786se8-014,20872se8-011,20625se8-012_allegra_lbl.pdf Accessed 06 March 2021.
Robbins DK, Castles MA, Pack DJ, Bhargava VO, Weir SJ. Dose proportionality and comparison of single and multiple dose pharmacokinetics of fexofenadine (MDL 16455) and its enantiomers in healthy male volunteers. Biopharm Drug Dispos. 1998;19:455–63.
Kusuhara H, Miura M, Yasui-Furukori N, Yoshida K, Akamine Y, Yokochi M, et al. Effect of coadministration of single and multiple doses of rifampicin on the pharmacokinetics of fexofenadine enantiomers in healthy subjects. Drug Metab Dispos. 2013;41:206–13.
Miura M, Uno T, Tateishi T, Suzuki T. Pharmacokinetics of fexofenadine enantiomers in healthy subjects. Chirality. 2007;19:223–7.
Pinto LSR, Vale GT, Moreira FL, Marques MP, Coelho EB, Cavalli RC, et al. Direct chiral LC-MS/MS analysis of fexofenadine enantiomers in plasma and urine with application in a maternal-fetal pharmacokinetic study. J Chrom B. 2020;1145:122094.
Pinto L, Moreira FL, Nardotto GHB, Cavalli RC, Moisés ECD, Duarte G, et al. Chiral discrimination of P-glycoprotein in parturient women: effect of fluoxetine on maternal-fetal fexofenadine pharmacokinetics. Pharm Res. 2020;37:131.
Chu X, Liao M, Shen H, Yoshida K, Zur AA, Arya V, et al. International transporter consortium. Clinical probes and endogenous biomarkers as substrates for transporter drug-drug interaction evaluation: perspectives from the international transporter consortium. Clin Pharmacol Ther. 2018;104(5):836–64.
Akamine Y, Miura M. An update on the clinical pharmacokinetics of fexofenadine enantiomers. Expert Opin Drug Metab Toxicol. 2018;14:429–34.
Weiss J, Dormann S-MG, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, Haefeli WE. Inhibition of P-Glycoprotein by newer antidepressants. J Pharmacol Exp Ther. 2003;305:197–204.
Argov M, Kashi R, Peer D, Margalit R. Treatment of resistant human colon cancer xenografts by a fluoxetine–doxorubicin combination enhances therapeutic responses comparable to an aggressive bevacizumab regimen. Cancer Lett. 2009;274:118–25.
Schrickx JA, Fink-Gremmels J. Inhibition of P-glycoprotein by psychotherapeutic drugs in a canine cell model. J Vet Pharmacol Therap. 2014;37:515–7.
Peer D, Dekel Y, Melikhov D, Margalit R. Fluoxetine inhibits multidrug resistance extrusion pumps and enhances responses to chemotherapy in syngeneic and in human xenograft mouse tumor models. Cancer Res. 2004;64:7562–9.
Miller RK, Wier PJ, Maulik D, Sant’agnese PA. Human placenta in vitro: characterization during 12 h of dual perfusion. Contrib Gynecol Obster. 1985;13:77–84.
Bapat P, Pinto LSR, Lubetsky A, Berger H, Koren G. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol. 2015;213:710.e1–6.
Bapat P, Pinto LSR, Lubetsky A, Aleksa K, Berger H, Koren G, Ito S. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost. 2016;14:1436–41.
Sudhakaran S, Rayner CR, Li J, Kong D, Gude NM, Nation RL. Inhibition of placental P-glycoprotein: impact on indinavir transfer to the foetus. Br J Clin Pharmacol. 2007;65:667–73.
Reynolds F, Knott C. Pharmacokinetics in pregnancy and placental drug transfer. Oxf Rev Reprod Biol. 1989;78–79:150–3.
Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–6.
Prouillac C, Lecoeur S. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos. 2010;38:1623–35.
Mao Q, Ganapathy V, Unadkat JD. Drug transport in the placenta. In: You G, Morris ME, editors. Drug transporters: molecular characterization and role in drug disposition. New Jersey: Wiley; 2014. p. 341–53.
Gavard L, Gil S, Peytavin G, Ceccaldi PF, Ferreira C, Farinotti R, et al. Placental transfer of lopinavir/ritonavir in the ex vivo human cotyledon perfusion model. Am J Obstet Gynecol. 2006;195:296–301.
Rahi M, Heikkinen T, Hakkola J, Hakala K, Wadelius M, Wadelius C, Laine K. Influence of adenosine triphosphate and ABCB1 (MDR1) genotype on the P-glycoprotein-dependent transfer of saquinavir in the dually perfused human placenta. Hum Exp Toxicol. 2008;1(27):65–71.
Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS. Role of P-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol. 2005;69:1869–78.
Gundogdu E, Alvarez IG, Karasulu E. Improvement of effect of water-in-oil microemulsion as an oral delivery system for fexofenadine: in vitro and in vivo studies. Int J Nanomedicine. 2011;6:1631–40.
Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289:963–9.
Garland M. Pharmacology of drug transfer across the placenta. Obstet Gynecol Clin N Am. 1998;25:21–42.
Sanofi-Aventis (2020). ALLEGRA™, fexofenadine hydrochloride: U.S. prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/20786se8-014,20872se8-011,20625se8-012_allegra_lbl.pdf. Accessed April 05, 2020.
Mölsä M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, Wadelius C, Laine K. Functional role of P-glycoprotein in the human blood-placental barrier. Clin Pharmacol Ther. 2005;2:123–31.
Ceccaldi P-F, Gavald L, Mandelbrot L, Rey E, Farinotti R, Treluyer J-M, Gil S. Functional role of P-glycoprotein and binding protein effect on the placental transfer of lopinavir/ritonavir in the ex vivo human perfusion model. Obstet Gynecol Int. 2009;2009:1–6.
Holcberg G, Sapir O, Tsadkin M, Huleihel M, Lazer S, Katz M, et al. Lack of interaction of digoxin and P-glycoprotein inhibitors, quinidine and verapamil in human placenta in vitro. Eur J Obst Gynecol Reprod Biol. 2003;109:133–7.
Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, et al. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug−drug interactions. J Med Chem. 2012;55:4740–63.
Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–81.
St-Pierre MV, Hagenbuch B, Ugele B, et al. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab. 2002;87:1856–63.
Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, et al. Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta. 2003;24:144–8.
Garg U, Frazee C. Therapeutic drug monitoring in infants and children. In: Clarke W, Dasgupta A, editors. Clinical challenges in therapeutic drug monitoring. Special populations, physiological conditions, and pharmacogenomics. Cambridge, MA: Elsevier; 2016. p. 1–361.
Financial Support
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; BEX6119/13–1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinto, L., Bapat, P., de Lima Moreira, F. et al. Chiral Transplacental Pharmacokinetics of Fexofenadine: Impact of P-Glycoprotein Inhibitor Fluoxetine Using the Human Placental Perfusion Model. Pharm Res 38, 647–655 (2021). https://doi.org/10.1007/s11095-021-03035-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03035-7