Abstract
Introduction
In the HELIOS trial, bendamustine/rituximab (BR) plus ibrutinib (BR-I) improved disease outcomes versus BR plus placebo in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Here, we describe the pharmacokinetic (PK) observations, along with modeling to further explore the interaction between ibrutinib and rituximab.
Methods
578 subjects were randomized to ibrutinib or placebo with BR (6 cycles). Ibrutinib PK samples and tumor measurements were obtained from all subjects; a subset was evaluated for bendamustine and rituximab PK. Population rituximab PK was assessed using nonlinear mixed-effects modeling.
Results
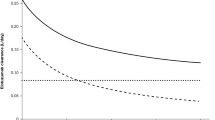
Dose-normalized plasma concentration-time bendamustine data were comparable between the arms. Systemic rituximab exposure was higher with BR-I versus BR; mean trough serum concentrations were 2- to 3-fold higher in the first three cycles and 1.2- to 1.7-fold higher subsequently. No relevant safety differences were observed. In the modeling, including treatment arm as a categorical covariate and tumor burden as a continuous time-varying covariate on overall rituximab clearance significantly improved fitting of the data.
Conclusions
BR-I led to higher dose-normalized systemic rituximab exposure versus BR and more rapid steady-state achievement. The modeling data suggest that rituximab disposition is, at least in part, target mediated. Determining the clinical significance of these findings requires further assessments.
Trial Registration
This study is registered at https://clinicaltrials.gov/ct2/show/NCT01611090.






Similar content being viewed by others
References
Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31:119–32.
Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23.
Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96.
IMBRUVICA® (ibrutinib) prescribing information. Sunnyvale: Pharmacyclics LLC; 2016.
IMBRUVICA® (ibrutinib) summary of product characteristics. Beerse: Janssen-Cilag International NV; 2016.
Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 2015;372:1430–40.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute–working group 1996 guidelines. Blood. 2008;111:5446–56.
Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German chronic lymphocytic leukemia study group. J Clin Oncol. 2011;29:3559–66.
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11.
RITUXAN® (rituximab) prescribing information. South San Francisco: Genentech, Inc.; 2016.
de Vries R, Huang M, Bode N, Jejurkar P, Jong J, Sukbuntherng J, et al. Bioanalysis of ibrutinib and its active metabolite in human plasma: selectivity issue, impact assessment and resolution. Bioanalysis. 2015;7:2713–24.
Marostica E, Sukbuntherng J, Loury D, de Jong J, de Trixhe XW, Vermeulen A, et al. Population pharmacokinetic model of ibrutinib, a Bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother Pharmacol. 2015;75:111–21.
Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user's guides (1989–2009). Ellicott City: Icon Development Solutions; 2009.
Li J, Zhi J, Wenger M, Valente N, Dmoszynska A, Robak T, et al. Population pharmacokinetics of rituximab in patients with chronic lymphocytic leukemia. J Clin Pharmacol. 2012;52:1918–26.
Müller C, Murawski N, Wiesen MH, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119:3276–84.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56:248–552.
Cramer P, Demirkan F, Fraser G, Pristupa A, Bartlett N, Dilhuydy M, et al. Ibrutinib increases the systemic exposure of rituximab: pharmacokinetic results from the HELIOS trial. Hematol Oncol. 2017;35(S2):232–3.
Lavezzi SM, de Jong J, Neyens M, et al. Modelling of rituximab clearance reduction due to ibrutinib co-administration. Proc PAGE 26. 2017:Abstr 7139 [www.page-meeting.org/?abstract=7139]. Accessed Sep 2017.
Gastonguay M. Full covariate models as an alternative to methods relying on statistical significance for inferences about covariate effects: a review of methodology and 42 case studies. Proc PAGE 20. 2011:Abstr 2229. [www.page-meeting.org/?abstract=2229]. Accessed Sep 2017.
Data on file. Janssen Research and Development, LLC. Raritan, NJ.
Golay J, Semenzato G, Rambaldi A, Foà R, Gaidano G, Gamba E, et al. Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. mAbs. 2013;5:826–37.
Poggesi I, Sardu ML, Marostica E, et al. Population pharmacokinetic-pharmacodynamic (PKPD) modeling of ibrutinib in patients with B-cell malignancies. Clin Cancer Res. 2015;21(17 Suppl):Abstr B19.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58.
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507.
Harrold JM, Straubinger RM, Mager DE. Combinatorial chemotherapeutic efficacy in non-Hodgkin lymphoma can be predicted by a signaling model of CD20 pharmacodynamics. Cancer Res. 2012;72:1632–41.
Yin A, Li J, Hurst D, Visich J. Population pharmacokinetics (PK) and association of PK and clinical outcomes of rituximab in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(suppl 15:e13108.
Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:995–1001.
Li J, Levi M, Charoin JE, et al. Rituximab exhibits a long halflife based on a population pharmacokinetic analysis in non-Hodgkin’s lymphoma (NHL) patients. Blood (ASH Annual Meeting Abstracts) 2008. Abstract 2371.
Tobinai K, Igarashi T, Itoh K, et al. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15:821–30.
Tout M, Gagez AL, Leprêtre S, Gouilleux-Gruart V, Azzopardi N, Delmer A, et al. Influence of FCGR3A-158V/F genotype and baseline CD20 antigen count on target-mediated elimination of rituximab in patients with chronic lymphocytic leukemia: a study of FILO group. Clin Pharmacokinet. 2017;56:635–47.
Dayde D, Ternant D, Ohresser M, Lerondel S, Pesnel S, Watier H, et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113:3765–72.
Liu C, Yu J, Li H, Liu J, Xu Y, Song P, et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther. 2017;101:657–66.
Buil-Bruna N, López-Picazo JM, Moreno-Jiménez M, Martín-Algarra S, Ribba B, Trocóniz IF. A population pharmacodynamic model for lactate dehydrogenase and neuron specific enolase to predict tumor progression in small cell lung cancer patients. AAPS J. 2014;16:609–19.
Acknowledgments and Disclosures
Dr. Laurie Orloski (independent medical writer) provided writing assistance and Dr. Namit Ghildyal (Janssen Research & Development, LLC.) provided additional editorial support for this manuscript. JdJ, MN MS, AH, MM, and IP are employees of Janssen Research & Development and hold stocks in the company; PC has received research grants from Hoffman-La Roche, Janssen, GlaxoSmithKline, Novartis, and Gilead and fees from AstraZeneca, Hoffmann La Roche, Janssen, Novartis, Astellas, and Mundipharma; GF has received research grants and fees from Janssen and Celgene and fees from Lundbeck; NB has received research grants from Janssen and Pharmacyclics; M-SD has received fees from Gilead, Janssen, and Roche; JL has received fees from Gilead, Janssen, Roche, and Abbvie; SR has received research grants and fees from Janssen, Roche, and Celgene and fees from Pharmacyclics; AG has received research grants and fees from Janssen, Pharmacyclics, Celgene, and Infinity, fees from Takeda and Acerta, and research grants from Genentech; SG has received research grants from Janssen and fees from Onyx and Seattle Genetics; SML, GDN, FD, AA, and OS have no conflicts of interest to disclose. The authors thank all the patients for their participation in this study and acknowledge the collaboration and commitment of all investigators and their staff.
Funding
Janssen Research and Development, LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lavezzi, S.M., de Jong, J., Neyens, M. et al. Systemic Exposure of Rituximab Increased by Ibrutinib: Pharmacokinetic Results and Modeling Based on the HELIOS Trial. Pharm Res 36, 93 (2019). https://doi.org/10.1007/s11095-019-2605-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2605-8