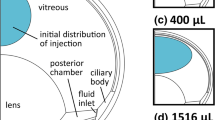
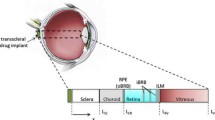
Abstract
Purpose
To extend the physiological features of the anatomically accurate model of the rabbit eye for intravitreal (IVT) and intracameral (IC) injections of macromolecules.
Methods
The computational fluid dynamic model of the rabbit eye by Missel (2012) was extended by enhancing the mixing in the anterior chamber with thermal gradient, heat transfer and gravity, and studying its effect on IC injections of hyaluronic acids. In IVT injections of FITC-dextrans (MW 10–157 kDa) the diffusion though retina was defined based on published in vitro data. Systematic changes in retinal permeability and convective transport were made, and the percentages of anterior and posterior elimination pathways were quantified. Simulations were compared with published in vivo data.
Results
With the enhanced mixing the elimination half-lives of hyaluronic acids after IC injection were 62–100 min that are similar to in vivo data and close to the theoretical value for the well-stirred anterior chamber (57 min). In IVT injections of FITC-dextrans a good match between simulations and in vivo data was obtained when the percentage of anterior elimination pathway was over 80%.
Conclusions
The simulations with the extended model closely resemble in vivo pharmacokinetics, and the model is a valuable tool for data interpretation and predictions.









Similar content being viewed by others
Abbreviations
- 2D/3D:
-
Two/three-dimensional
- AH:
-
Aqueous humor
- AMD:
-
Age-related macular degeneration
- ATM :
-
Amount of macromolecule eliminated through trabecular meshwork with aqueous drainage
- AUCa :
-
Area under the concentration curve in the aqueous humor
- Ca :
-
Mean concentration of macromolecule in the aqueous humor (anterior chamber)
- CFD :
-
Computational fluid dynamics
- Cv :
-
Mean concentration of macromolecule in the vitreous
- D:
-
Diffusion coefficient of macromolecule in water
- Dret :
-
Apparent diffusion coefficient of macromolecule in retina
- f:
-
Aqueous humor flow rate
- FEM:
-
Finite element modeling
- IC:
-
Intracameral
- IOP :
-
Intraocular pressure
- IVT:
-
Intravitreal
- kac :
-
Elimination rate constant of macromolecule from the anterior chamber
- kv :
-
Elimination rate constant of macromolecule from the vitreous
- MRI:
-
Magnetic resonance imaging
- MW:
-
Molecular weight
- Papp :
-
Apparent permeability coefficient of macromolecule in membrane
- PK:
-
Pharmacokinetics
- rH :
-
Hydrodynamic radius
- RPE:
-
Retinal pigment epithelium
- t1/2 :
-
Elimination half-life
- TM :
-
Trabecular meshwork
- Va :
-
Volume of the anterior chamber
- Vv :
-
Volume of the vitreous
References
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):106–16.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–6.
Hutton-Smith LA, Gaffney EA, Byrne HM, Maini PK, Gadkar K, Mazer NA. Ocular pharmacokinetics of therapeutic antibodies given by intravitreal injection: estimation of retinal permeabilities using a 3-compartment semi-mechanistic model. Mol Pharm. 2017;14(8):2690–6.
Del Amo EM, Rimpelä AK, Heikkinen E, Kari OK, Ramsay E, Lajunen T, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–85.
Cunha-Vaz JG, Maurice DM. The active transport of fluorescein by the retinal vessels and the retina. J Physiol. 1967;191(3):467–86.
Araie M, Maurice DM. The loss of fluorescein, fluorescein glucuronide and fluorescein isothiocyanate dextran from the vitreous by the anterior and retinal pathways. Exp Eye Res. 1991;52(1):27–39.
Tan LE, Orilla W, Hughes PM, Tsai S, Burke JA, Wilson CG. Effects of vitreous liquefaction on the intravitreal distribution of sodium fluorescein, fluorescein dextran, and fluorescent microparticles. Invest Ophthalmol Vis Sci. 2011;52(2):1111–8.
Kim H, Lizak MJ, Tansey G, Csaky KG, Robinson MR, Yuan P, et al. Study of ocular transport of drugs released from an intravitreal implant using magnetic resonance imaging. Ann Biomed Eng. 2005;33(2):150–64.
Tojo KJ, Ohtori A. Pharmacokinetic model of intravitreal drug injection. Math Biosci. 1994;123(1):59–75.
Friedrich S, Cheng YL, Saville B. Finite element modeling of drug distribution in the vitreous humor of the rabbit eye. Ann Biomed Eng. 1997;25(2):303–14.
Missel PJ. Simulating intravitreal injections in anatomically accurate models for rabbit, monkey, and human eyes. Pharm Res. 2012;29(12):3251–72.
Friedrich S, Cheng YL, Saville B. Drug distribution in the vitreous humor of the human eye: the effects of intravitreal injection position and volume. Curr Eye Res. 1997;16(7):663–9.
Tojo K, Nakagawa K, Morita Y, Ohtori A. A pharmacokinetic model of intravitreal delivery of ganciclovir. Eur J Pharm Biopharm. 1999;47(2):99–104.
Stay MS, Xu J, Randolph TW, Barocas VH. Computer simulation of convective and diffusive transport of controlled-release drugs in the vitreous humor. Pharm Res. 2003;20(1):96–102.
Balachandran RK, Barocas VH. Computer modeling of drug delivery to the posterior eye: effect of active transport and loss to choroidal blood flow. Pharm Res. 2008;25(11):2685–96.
Kotha S, Murtomäki L. Virtual pharmacokinetic model of human eye. Math Biosci. 2014;253:11–8.
Friedrich S, Saville B, Cheng YL. Drug distribution in the vitreous humor of the human eye: the effects of aphakia and changes in retinal permeability and vitreous diffusivity. J Ocul Pharmacol Ther. 1997;13(5):445–59.
Kathawate J, Acharya S. Computational modeling of intravitreal drug delivery in the vitreous chamber with different vitreous substitutes. Int J Heat Mass Transf. 2008;51(23):5598–609.
Balachandran RK, Barocas VH. Contribution of saccadic motion to intravitreal drug transport: theoretical analysis. Pharm Res. 2011;28(5):1049–64.
Missel PJ, Horner M, Muralikrishnan R. Simulating dissolution of intravitreal triamcinolone acetonide suspensions in an anatomically accurate rabbit eye model. Pharm Res. 2010;27(8):1530–46.
Tojo K. A pharmacokinetic model for ocular drug delivery. Chem Pharm Bull. 2004;52(11):1290–4.
Ueda K, Ohtori A, Tojo K. Effects of pathological conditions on ocular pharmacokinetics of antimicrobial drugs. Chem Pharm Bull. 2010;58(10):1301–5.
Shikamura Y, Ohtori A, Tojo K. Drug penetration of the posterior eye tissues after topical instillation: in vivo and in silico simulation. Chem Pharm Bull. 2011;59(10):1263–7.
Park J, Bungay PM, Lutz RJ, Augsburger JJ, Millard RW, Sinha Roy A, et al. Evaluation of coupled convective-diffusive transport of drugs administered by intravitreal injection and controlled release implant. J Control Release. 2005;105(3):279–95.
Krishnamoorthy MK, Park J, Augsburger JJ, Banerjee RK. Effect of retinal permeability, diffusivity, and aqueous humor hydrodynamics on pharmacokinetics of drugs in the eye. J Ocul Pharmacol Ther. 2008;24(3):255–67.
Wyatt HJ. Ocular pharmacokinetics and convectional flow: evidence from spatio-temporal analysis of mydriasis. J Ocul Pharmacol Ther. 1996;12(4):441–59.
Wyatt HJ. Modelling transport in the anterior segment of the eye. Optom Vis Sci. 2004;81(4):272–82.
Canning CR, Greaney MJ, Dewynne JN, Fitt AD. Fluid flow in the anterior chamber of a human eye. IMA J Math Appl Med Biol. 2002;19(1):31–60.
Heys JJ, Barocas VH. A boussinesq model of natural convection in the human eye and the formation of Krukenberg's spindle. Ann Biomed Eng. 2002;30(3):392–401.
Johnson F, Maurice D. A simple method of measuring aqueous humor flow with intravitreal fluoresceinated dextrans. Exp Eye Res. 1984;39(6):791–805.
Araie M. Time change of rabbit aqueous flow under influence of adrenergic drugs. Exp Eye Res. 1985;41(3):391–403.
Pitkänen L, Ranta V, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46(2):641–6.
Cantrill HL, Pederson JE. Experimental retinal detachment. VI. The permeability of the blood-retinal barrier. Arch Ophthalmol. 1984;102(5):747–51.
Hirata A, Watanabe S, Kojima M, Hata I, Wake K, Taki M, et al. Computational verification of anesthesia effect on temperature variations in rabbit eyes exposed to 2.45 GHz microwave energy. Bioelectromagnetics. 2006;27(8):602–12.
Ng EYK, Ooi EH. FEM simulation of the eye structure with bio-heat analysis. Comput Methods Prog Biomed. 2006;82(3):268–76.
Lagendijk JJ. A mathematical model to calculate temperature distributions in human and rabbit eyes during hyperthermic treatment. Phys Med Biol. 1982;27(11):1301–11.
Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55(3):383–417.
Karampatzakis A, Samaras T. Numerical model of heat transfer in the human eye with consideration of fluid dynamics of the aqueous humour. Phys Med Biol. 2010;55(19):5653–65.
Schwartz B, Feller MR. Temperature gradients in the rabbit eye. Investig Ophthalmol. 1962;1:513–21.
Lorget F, Parenteau A, Carrier M, Lambert D, Gueorguieva A, Schuetz C, et al. Characterization of the pH and temperature in the rabbit, pig, and monkey eye: key parameters for the development of long-acting delivery ocular strategies. Mol Pharm. 2016;13(9):2891–6.
Laurent TC, Ryan M, Pietruszkiewicz A. Fractionation of hyaluronic acid. The polydispersity of hyaluronic acid from the bovine vitreous body. Biochim Biophys Acta. 1960;42:476–85.
Amirpour N, Karamali F, Razavi S, Esfandiari E, Nasr-Esfahani MH. A proper protocol for isolation of retinal pigment epithelium from rabbit eyes. Adv Biomed Res. 2014;3:4.
Zhou J, He S, Zhang N, Spee C, Zhou P, Ryan SJ, et al. Neutrophils compromise retinal pigment epithelial barrier integrity. J Biomed Biotechnol. 2010;2010:289360.
Braeckmans K, Peeters L, Sanders NN, De Smedt SC, Demeester J. Three-dimensional fluorescence recovery after photobleaching with the confocal scanning laser microscope. Biophys J. 2003;85(4):2240–52.
Laurent UB, Fraser JR. Turnover of hyaluronate in the aqueous humour and vitreous body of the rabbit. Exp Eye Res. 1983;36(4):493–503.
Maurice DM. Injection of drugs into the vitreous body. In: Leopold I, Burns R, editors. Symposium on ocular therapy, vol. 9. London: Wiley; 1976. p. 59–72.
Maurice DM, Mishima S. Ocular pharmacology. In: Sears M, editor. Handbook of experimental pharmacology. Berlin-Heidelberg: Springer-Verlag; 1984. p. 16–119.
Yablonski ME, Hayashi M, Cook DJ, Chubak G, Sirota M. Fluorophotometric study of intravenous carbonic anhydrase inhibitors in rabbits. Invest Ophthalmol Vis Sci. 1987;28(12):2076–82.
Hutton-Smith LA, Gaffney EA, Byrne HM, Maini PK, Schwab D, Mazer NA. A mechanistic model of the intravitreal pharmacokinetics of large molecules and the pharmacodynamic suppression of ocular vascular endothelial growth factor levels by ranibizumab in patients with neovascular age-related macular degeneration. Mol Pharm. 2016;13(9):2941–50.
Schmitt W. Estimation of intra-vitreal half-lifes in the rabbit eye with semi-mechanistic equations. Pharm Res. 2017;34(1):49–57.
Maurice DM. Protein dynamics in the eye studied with labelled proteins. Am J Ophthalmol. 1959;47(1 Pt 2):361–8.
Haghjou N, Abdekhodaie MJ, Cheng Y. Retina-choroid-sclera permeability for ophthalmic drugs in the vitreous to blood direction: quantitative assessment. Pharm Res. 2013;30(1):41–59.
Zhao M, Hejkal JJ, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Invest Ophthalmol Vis Sci. 2010;51(6):3145–51.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 2.13 mb)
Rights and permissions
About this article
Cite this article
Lamminsalo, M., Taskinen, E., Karvinen, T. et al. Extended Pharmacokinetic Model of the Rabbit Eye for Intravitreal and Intracameral Injections of Macromolecules: Quantitative Analysis of Anterior and Posterior Elimination Pathways. Pharm Res 35, 153 (2018). https://doi.org/10.1007/s11095-018-2435-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2435-0