ABSTRACT
Purpose
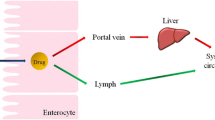
To develop novel bioactive-chylomicrons to solve oral delivery obstacles of Berberine chloride and target the lymphatic system.
Methods
Berberine-loaded bioactive-chylomicrons were prepared and underwent full in vitro characterization. Intestinal permeability was appraised via both non-everted gut sac model and Caco-2 cell model. Furthermore, Bioactive-chylomicrons’ cellular uptake and distribution were examined by laser scanning confocal microscopy. Finally, a novel chylomicron flow-blockage assay on tissue and cellular levels were elaborated to assess the lymphatic targeting ability.
Results
Berberine-loaded chylomicrons showed spherical vesicles of size (175.6 nm), PDI (0.229), zeta potential (−16 .6 mV) and entrapment efficiency (95.5%). Ex-vivo intestinal permeability studies demonstrated 10.5 fold enhancement in permeability of Berberine-loaded chylomicrons over free Berberine. Moreover, Caco-2 studies revealed significant improvement in chylomicrons’ permeability and cellular uptake. Furthermore, confocal microscopy analyses revealed 2 fold increase in berberine-loaded chylomicrons’ intracellular fluorescence. Lymphatic targeting models were successfully elaborated using cycloheximide protein synthesis inhibitor. Such models demonstrated 47 and 27.5% reduction in ex-vivo and Caco-2 permeability respectively. Finally, a good rank order correlation was established between different permeability assessment techniques.
Conclusion
The findings shed the light on the underlying mechanisms of Berberine bioavailability improvement. Consequently, berberine-loaded chylomicrons could be considered as promising bioactive-nanocarriers for Berberine lymphatic targeting and bioavailability improvement.









Similar content being viewed by others
Abbreviations
- BER:
-
Berberine chloride
- BSA:
-
Bovine serum albumin
- CM:
-
Chylomicron
- CHX:
-
Cycloheximide
- LSCM:
-
Laser scanning confocal microscopy
- P-gp:
-
p-glycoprotein
- PDI:
-
Polydispersity index
- ROI:
-
Region of interest
- CMo :
-
Unmodified chylomicron (surfactant free)
REFERENCES
Nguyen TX, Huang L, Liu L, Elamin Abdalla AM, Gauthier M, Yang G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J Mater Chem B. 2014;2:7149–59.
Xue M, Yang MX, Zhang W, Li XM, Gao DH, Ou ZM, et al. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomedicine. 2013;8:4677–87.
Ortiz LMG, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19:12349–67.
Goto H, Kariya R, Shimamoto M, Kudo E, Taura M, Katano H, et al. Antitumor effect of berberine against primary effusion lymphoma via inhibition of NF-B pathway. Cancer Sci. 2012;103:775–81.
Zhang Z, Chen Y, Deng J, Jia X, Zhou J, Lv H. Solid dispersion of berberine–phospholipid complex/TPGS 1000/SiO2: preparation, characterization and in vivo studies. Int J Pharm. 2014;465:1–11.
Sailor G, Seth AK, Parmar G, Chauhan S, Javia A. Formulation and in vitro evaluation of berberine containing liposome optimized by 32 full factorial designs. J Appl Pharm Sci. 2015;5:23–8.
Liu Y-T, Hao H-P, Xie H-G, Lai L, Wang Q, Liu C-X, et al. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos. 2010;38:1779–84.
Chen W, Miao Y-Q, Fan D-J, Yang S-S, Lin X, Meng L-K, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12:705–11.
Souza CR, Oliveira HR, Pinheiro WM, Biswaro LS, Azevedo RB, Gomes AJ, et al. Gold nanoparticle and berberine entrapped into hydrogel matrix as drug delivery system. J Biomater Nanobiotechnol. 2015;6:53–63.
Interaction L. New oral delivery system to improve absorption of berberine: likely interaction of cationized chitosan with PG-P pump. Int J Drug Deliv Technol. 2014;5:33–42.
Fan Z, Wu J, Fang X, Sha X. A new function of vitamin E-TPGS in the intestinal lymphatic transport of lipophilic drugs: enhancing the secretion of chylomicrons. Int J Pharm. 2013;445:141–7.
Ghosh S, Roy T. Nanoparticulate drug-delivery systems: lymphatic uptake and its gastrointestinal applications. J Appl Pharm Sci. 2014;4:130–23.
Van Greevenbroek MMJ, De Bruin TWA. Chylomicron synthesis by intestinal cells in vitro and in vivo. Atherosclerosis. 1998;141:9–16.
Dahan A, Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24:381–8.
Sek L, Porter CJH, Charman WN. Characterisation and quantification of medium chain and long chain triglycerides and their in vitro digestion products, by HPTLC coupled with in situ densitometric analysis. J Pharm Biomed Anal. 2001;25(3–4):651–61.
Gershkovich P, Hoffman A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci. 2005;26:394–404.
Dierling AM, Cui Z. Targeting primaquine into liver using chylomicron emulsions for potential vivax malaria therapy. Int J Pharm. 2005;303:143–52.
Jain V, Nath B, Gupta GK, Shah PP, Siddiqui MA, Pant AB, et al. Galactose-grafted chylomicron-mimicking emulsion: evaluation of specificity against HepG-2 and MCF-7 cell lines. J Pharm Pharmacol. 2009;61:303–10.
Freag MS, Elnaggar YSR, Abdallah OY. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: optimization and ex-vivo permeation. Int J Nanomedicine. 2013;8:2385–97.
Bothiraja C, Pawar A, Deshpande G. Ex-vivo absorption study of a nanoparticle based novel drug delivery system of vitamin D3 (Arachitol NanoTM) using everted intestinal sac technique. J Pharm Investig. 2016;46:425–32.
Lin YC, Kuo JY, Hsu CC, Tsai WC, Li WC, Yu MC, et al. Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int J Pharm. 2013;441:381–8.
Hanchinalmath JV, Londonkar R. Cytotoxic and apoptosis-inducing effect of luteolin isolated from feronia limonia on hepg2 cells. Biolife. 2012;2:1287–92.
Tormalehto S, Monkkonen H, Asunmaa K, Niemi R, Auriola S, Vepsalainen J, et al. C ellular uptake and metabolism of clodronate and its derivatives in Caco-2 cells: a possible correlation with bisphosphonate-induced gastrointestinal. Cell Prolif. 2003;19:23–9.
Tang C, Wu XD, Yu YM, Duan H, Zhou J, Xu L. Cell extraction combined with off-line HPLC for screening active compounds from Coptis chinensis. Biomed Chromatogr. 2016;30:658–62.
Sera TL, Paulo W, Vilma JO, Perkins E, Parke D, Holy J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother Pharmacol. 2008;61:1007–18.
Ismail M. The use of Caco-2 cells as an in vitro method to study bioavailability of iron. Mal J Nutr. 1999;5:31–45.
Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J Pharmacol Exp Ther. 2003;306:355–62.
Zidan AS, Spinks CB, Habib MJ, Khan MA. Formulation and transport properties of tenofovir loaded liposomes through Caco-2 cell model. J Liposome Res. 2013;2104:1–9.
Field EJ, Albright E, Mathur SN. Regulation of trigiyceride-rich Lipoproteinsecretion by fatty acids in Caco-2 cells. J Lipid Res. 1988;29:1427–37.
Paliwal R, Paliwal SR, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int J Pharm. 2009;380:181–8.
Reix N, Parat A, Seyfritz E, Van Der Werf R, Epure V, Ebel N, et al. In vitro uptake evaluation in Caco-2 cells and in vivo results in diabetic rats of insulin-loaded PLGA nanoparticles. Int J Pharm. 2012;437:213–20.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91:193–7.
Rege BD, Kao JPY, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16:237–46.
Seeballuck F, Lawless E, Ashford MB, O’Driscoll CM. Stimulation of triglyceride-rich lipoprotein secretion by polysorbate 80: in vitro and in vivo correlation using caco-2 cells and a cannulated rat intestinal lymphatic model. Pharm Res. 2004;21:2320–6.
Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2012;64:280–9.
Elsheikh MA, Elnaggar YSR, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: optimization and in vivo appraisal. Int J Nanomedicine. 2012;7:3787–802.
Yang H-T, Wang G-J. Transport and uptake characteristics of a new derivative of berberine (CPU-86017) by human intestinal epithelial cell line: caco-2. Acta Pharmacol Sin. 2003;24:1185–91.
Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803.
Acknowledgements
The authors are deeply grateful to Centre of Excellence for Research in Regenerative Medicine and its Applications (CERRMA) at Faculty of Medicine, Alexandria University, Egypt where Cell culture experiments were conducted. The authors also acknowledge the Medical Research Centre I at the Faculty of Medicine, Alexandria University. Egypt where the HPLC analyses were performed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 63 kb)
Rights and permissions
About this article
Cite this article
Elsheikh, M.A., Elnaggar, Y.S.R., Otify, D.Y. et al. Bioactive-Chylomicrons for Oral Lymphatic Targeting of Berberine Chloride: Novel Flow-Blockage Assay in Tissue-Based and Caco-2 Cell Line Models. Pharm Res 35, 18 (2018). https://doi.org/10.1007/s11095-017-2307-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2307-z