ABSTRACT
Purpose
Multifractal geometry has become a powerful tool to describe complex structures in many fields. Our first aim was to combine imaging and multifractal analysis to better understand the microstructure of pharmaceutical extrudates. A second objective was to study erosion/dispersion behavior of the formulations because it would condition release of any drug.
Methods
Different formulations containing a lipid, a polymer and different silica based inorganic carriers were produced by hot-melt extrusion at various screw speeds. Multifractal analysis was based on scanning electron microscopy/energy dispersive X-Ray spectroscopy images. This microstructural analysis was complemented with dynamic optical imaging of formulation erosion/dispersion behavior.
Results
Multifractal analysis indicated that inorganic carrier type and concentration as well as the screw speed affected the microstructure of the extrudates. The aqueous erosion/dispersion study showed that only the type and concentration of inorganic carrier were important.
Conclusions
The use of microstructural and dispersion analysis appeared to be complementary to better characterize and understand complex formulations obtained by hot-melt extrusion.









Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- EDS:
-
Electron dispersive X-Ray spectroscopy
- HME:
-
Hot-melt extrusion
- PVPVA:
-
Polyvinylpyrrolidone-vinyl acetate
- SEM:
-
Scanning electron microscopy
- Si:
-
Silicon
REFERENCES
Pippa N, Dokoumetzidis A, Demetzos C, Macheras P. On the ubiquitous presence of fractals and fractal concepts in pharmaceutical sciences: a review. Int J Pharm. 2013;456(2):340–52.
Lopes R, Betrouni N. Fractal and multifractal analysis: a review. Med Image Anal. 2009;13(4):634–49.
Cheng Q. Multifractality and spatial statistics. Comput Geosci. 1999;25(9):949–61.
Gómez-Carracedo A, Alvarez-Lorenzo C, Coca R, Martínez-Pacheco R, Concheiro A, Gómez-Amoza JL. Fractal analysis of SEM images and mercury intrusion porosimetry data for the microstructural characterization of microcrystalline cellulose-based pellets. Acta Mater. 2009;57(1):295–303.
Valentini L, Artioli G, Voltolini M, Dalconi MC. Multifractal analysis of calcium silicate hydrate (C-S-H) mapped by X-ray diffraction Microtomography. Jennings H, editor. J Am Ceram Soc. 2012;95(8):2647–52.
Mendoza F, Verboven P, Ho QT, Kerckhofs G, Wevers M, Nicolaï B. Multifractal properties of pore-size distribution in apple tissue using X-ray imaging. J Food Eng. 2010;99(2):206–15.
Dathe A, Tarquis AM, Perrier E. Multifractal analysis of the pore- and solid-phases in binary two-dimensional images of natural porous structures. Geoderma. 2006;134(3–4):318–26.
Klang V, Valenta C, Matsko NB. Electron microscopy of pharmaceutical systems. Micron. 2013. p. 45–74.
Park C-W, Rhee Y-S, Vogt FG, Hayes D, Zwischenberger JB, DeLuca PP, et al. Advances in microscopy and complementary imaging techniques to assess the fate of drugs ex vivo in respiratory drug delivery: an invited paper. Adv Drug Deliv Rev. 2012;64(4):344–56.
Thibert R, Akbarieh M, Tawashi R. Application of fractal dimension to the study of the surface ruggedness of granular solids and excipients. J Pharm Sci. 1988;77(8):724–6.
Gupta MK, Vanwert A, Bogner RH. Formation of physically stable amorphous drugs by milling with neusilin. J Pharm Sci. 2003;92(3):536–51.
Adler C, Schönenberger M, Teleki A, Kuentz M. Molecularly designed lipid microdomains for solid dispersions using a polymer/inorganic carrier matrix produced by hot-melt extrusion. Int J Pharm. 2016;499(1–2):90–100.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23–24):1068–75.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60.
Ali S, Kolter K. Challenges and opportunities in oral formulation development. Am Pharm Rev. 2012;15(7).
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33(10):1043–57.
Stanković M, Frijlink HW, Hinrichs WLJ. Polymeric formulations for drug release prepared by hot melt extrusion: application and characterization. Drug Discov Today. 2015;1–12.
Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol. 2005;57(5):533–46.
Harmon P, Galipeau K, Xu W, Brown C, Wuelfing WP. Mechanism of dissolution-induced Nanoparticle formation from a Copovidone-based amorphous solid dispersion. Mol Pharm Am Chem Soc. 2016;13(5):1467–81.
Bialleck S, Rein H. Drug release mechanisms of hot-melt extruded starch-based pellets. Starch/Staerke. 2012;64(5):408–19.
Serajuddln ATM. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058–66.
Pudlas M, Kyeremateng SO, Williams LAM, Kimber JA, van Lishaut H, Kazarian SG, et al. Analyzing the impact of different excipients on drug release behavior in hot-melt extrusion formulations using FTIR spectroscopic imaging. Eur J Pharm Sci. 2015;67:21–31.
Bravo-Osuna I, Ferrero C, Jiménez-Castellanos MR. Drug release behaviour from methyl methacrylate-starch matrix tablets: effect of polymer moisture content. Eur J Pharm Biopharm. 2008;69(1):285–93.
Posadas AND, Giménez D, Bittelli M, Vaz CMP, Flury M. Multifractal characterization of soil particle-size distributions. Soil Sci Soc Am J. 2001;65(5):1361.
Halsey TC, Jensen MH, Kadanoff LP, Procaccia I, Shraiman BI. Fractal measures and their singularities: the characterization of strange sets. Nucl Phys B. 1987;2(C):501–11.
Angulo J, Esquivel F. Multifractal Dimensional Dependence Assessment Based on Tsallis Mutual Information. Entropy. Multidisciplinary Digital Publishing Institute; 2015;17(8):5382–401.
Turcotte DL. Fractal models in the earth sciences. Tectonophysics. 1993;227(1–4):234.
Bumm SH. Mixing studies and simulation of compounding chopped fiber and silica filler into thermoplastics in a modular co-rotating twin screw extruder. Akron: University of Akron; 2010.
Aeroperl Granulated Fumed Oxids-Technical Information 1341. :Evonik Industries.
Aerosil and Aeroperl Colloidal Silicon Dioxid for Pharmaceuticals- Technical Information TI 1281. :Evonik Industries.
Kadajji VG, Betageri G V. Water soluble polymers for pharmaceutical applications. Polymers. 2011. p. 1972–2009.
Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol Today. 2000;3(6):198–204.
Colombo P, Bettini R, Santi P, De Ascentiis A, Peppas NA. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J Control Release. 1996;39(2–3):231–7.
Joyce P, Tan A, Whitby CP, Prestidge CA. The role of porous nanostructure in controlling lipase-mediated digestion of lipid loaded into silica particles. Langmuir Am Chem Soc. 2014;30(10):2779–88.
Van Speybroeck M, Williams HD, Nguyen T-H, Anby MU, Porter CJH, Augustijns P. Incomplete desorption of liquid excipients reduces the in vitro and in vivo performance of self-emulsifying drug delivery systems solidified by adsorption onto an inorganic Mesoporous carrier. Mol Pharm. 2012;9(9):2750–60.
Florite. :Tomita Pharmaceutical Co., Ltd. www.tomitaph.co.jp/english/data/FLORITE.pdf.
Author information
Authors and Affiliations
Corresponding author
APPENDIX A
APPENDIX A
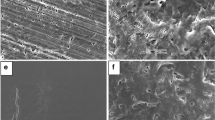
SEM pictures of the used adsorbent powders are presented in this Appendix A to illustrate their physical variety.
SEM pictures of fumed Aerosil 300 (a), and Aerosil R 972 (b). Aerosil fumed silicates are very fine powders composed of aggregated and agglomerated primary particles. Primary particles could not be identified due to their very small size (< 50 nm) (30).
SEM pictures of Aeroperl 300 (a, d), Florite R (b, e), and Neusilin US2 (c, f). Aeroperl 300 spherical granules have a rather smooth surface. Florite R particles exhibit irregular shape while the pore structure can be viewed as petaloid. Finally, the spherical Neusilin US2 particles display some porosity on their surface compared to Aeroperl 300.
Rights and permissions
About this article
Cite this article
Adler, C., Teleki, A. & Kuentz, M. Multifractal Characterization of Pharmaceutical Hot-Melt Extrudates. Pharm Res 34, 321–332 (2017). https://doi.org/10.1007/s11095-016-2064-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2064-4