Abstract
Purpose
The objective of this study was to use a recently developed nasal dissolution, absorption, and clearance (DAC) model to evaluate the extent to which suspended drug particle size influences nasal epithelial drug absorption for a spray product.
Methods
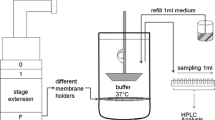
Computational fluid dynamics (CFD) simulations of mucociliary clearance and drug dissolution were used to calculate total and microscale epithelial absorption of drug delivered with a nasal spray pump. Ranges of suspended particle sizes, drug solubilities, and partition coefficients were evaluated.
Results
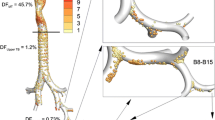
Considering mometasone furoate as an example, suspended drug particle sizes in the range of 1-5 μm did not affect the total nasal epithelial uptake. However, the microscale absorption of suspended drug particles with low solubilities was affected by particle size and this controlled the extent to which the drug penetrated into the distal nasal regions.
Conclusions
The nasal-DAC model was demonstrated to be a useful tool in determining the nasal exposure of spray formulations with different drug particle sizes and solubilities. Furthermore, the model illustrated a new strategy for topical nasal drug delivery in which drug particle size is selected to increase the region of epithelial surface exposure using mucociliary clearance while minimizing the drug dose exiting the nasopharynx.












Similar content being viewed by others
Abbreviations
- AEF:
-
Absorption enhancement factor
- ASL:
-
Airway surface liquid
- CFD:
-
Computational fluid dynamics
- DAC:
-
Dissolution-absorption-clearance
- MF:
-
Mometasone furoate
- PK:
-
Pharmacokinetic
References
Li BV, Jin F, Lee SL, Bai T, Chowdhury B, Caramenico HT, et al. Bioequivalence for locally acting nasal spray and nasal aerosol products: standard development and generic approval. AAPS J. 2013;15(3):875–83.
Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the united states food and drug administration. Ann Pharmacother. 2009;43(10):1583–97.
Suman JD, Laube BL, Dalby R. Validity of in vitro tests on aqueous spray pumps as surrogates for nasal deposition, absorption, and biologic response. J Aerosol Med. 2006;19(4):510–21.
Turker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004;26(3):137–42.
Pires Ai, Fortuna A, Alves G, Fal1 ao Ai. Intranasal Drug Delivery: How, Why and What For? Journal of Pharmacy and Pharmaceutical Sciences. 2009;12(3):288-311.
Buttini F, Miozzi M, Balducci AG, Royall PG, Brambilla G, Colombo P, et al. Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers. Int J Pharm. 2014;465(1):42–51.
Arora D, Shah KA, Halquist MS, Sakagami M. In vitro aqueous fluid-capacity-limited dissolution testing of respirable aerosol drug particles generated from inhaler products. Pharm Res. 2010;27(5):786–95.
Schroeter JD, Kimbell JS, Asgharian B. Analysis of particle deposition in the turbinate and olfactory regions using a human nasal computational fluid dynamics model. J Aerosol Med. 2006;19(3):301–13.
Shi H, Kleinstreuer C, Zhang Z. Modeling of inertial particle transport and deposition in human nasal cavities with wall roughness. J Aerosol Sci. 2007;38(4):398–419.
Liu Y, Matida EA, Johnson MR. Experimental measurements and computational modeling of aerosol deposition in the Carleton-civic standardized human nasal cavity. J Aerosol Sci. 2010;41(6):569–86.
Inthavong K, Ge Q, Se CMK, Yang W, Tu JY. Simulation of sprayed particle deposition in a human nasal cavity including a nasal spray device. J Aerosol Sci. 2011;42(2):100–13.
Schroeter JD, Garcia GJM, Kimbell JS. Effects of surface smoothness on inertial particle deposition in human nasal models. J Aerosol Sci. 2011;42(1):52–63.
Xi J, Si X, Kim JW, Berlinski A. Simulation of airflow and aerosol deposition in the nasal cavity of a 5-year-Old child. J Aerosol Sci. 2011;42(3):156–73.
Kelly JT, Asgharian B, Kimbell JS, Wong BA. Particle deposition in human nasal airway replicas manufactured by different methods. Part I: inertial regime particles. Aerosol Sci Technol. 2004;38(11):1063–71.
Kelly JT, Asgharian B, Kimbell JS, Wong BA. Particle deposition in human nasal airway replicas manufactured by different methods. Part II: ultrafine particles. Aerosol Sci Technol. 2004;38(11):1072–9.
Garcia GJM, Tewksbury EW, Ba W, Kimbell JS. Interindividual Variability in nasal filtration as a function of nasal cavity geometry. J Aerosol Med Pulm Drug Deliv. 2009;22(2):139–55.
Shah SA, Dickens CJ, Ward DJ, Banaszek AA, George C, Horodnik W. Design of experiments to optimize an in vitro cast to predict human nasal drug deposition. J Aerosol Med Pulm Drug Deliv. 2014;26:1–9.
Shah SA, Berger RL, McDermott J, Gupta P, Monteith D, Connor A, et al. Regional deposition of mometasone furoate nasal spray suspension in humans. Allergy Asthma Proc. 2015;36(1):48–57.
Kimbell JS, Subramaniam RP. Use of computational fluid dynamics models for dosimetry of inhaled gases in the nasal passages. Inhal Toxicol. 2001;13(5):325–34.
Zhang Z, Kleinstreuer C, Kim CS. Transport and uptake of MTBE and ethanol vapors in a human upper airway model. Inhal Toxicol. 2006;18(3):169–84.
Tian G, Longest PW. Development of a CFD boundary condition to model transient vapor absorption in the respiratory airways. J Biomech Eng. 2010;132(5):051003.
Tian G, Longest PW. Transient absorption of inhaled vapors into a multilayer mucus-tissue-blood system. Ann Biomed Eng. 2010;38(2):517–36.
Bush ML, Frederick CB, Kimbell JS, Ultman JS. A CFD PBPK Hybrid model for simulating Gas and vapor uptake in the Rat nose. Toxicol Appl Pharmacol. 1998;150(1):133–45.
Rygg A, Longest PW. Absorption and Clearance of Pharmaceutical Aerosols in the Human Nose: Development of a CFD Model. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2015; In press.
Xi J, Longest PW. Numerical Predictions of submicrometer aerosol deposition in the nasal cavity using a novel drift flux approach. Int J Heat Mass Transf. 2008;51(23-24):5562–77.
Fry FA, Black A. Regional deposition and clearance of particles in the human nose. J Aerosol Sci. 1973;4(2):113–24.
Illum L. Nasal drug delivery - possibilities, problems and solutions. J Control Release. 2003;87(1-3):187–98.
Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1-2):146–57.
Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal Mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57(11):1640–65.
Beule AG. Physiology and Pathophysiology of the Paranasal Sinuses. GMS Current Topics in Otorhinolaryngology - Head and Neck Surgery. 2010; 9
Quraishi MS, Jones NS, Mason J. The rheology of nasal mucus: a review. Clin Otolaryngol Allied Sci. 1998;23(5):403–13.
McLean J, Bacon J, Mathews K, Thrall J, Banas J, Hedden J, et al. Distribution and clearance of radioactive aerosol on the nasal mucosa. Rhinology. 1984;22(1):65–75.
Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective - a review. Drug Deliv Transl Res. 2013;3(1):42–62.
Cu Y, Saltzman WM. Mathematical modeling of molecular diffusion through mucus. Adv Drug Deliv Rev. 2009;61(2):101–14.
Gulliver JS. Introduction to chemical transport in the environment. Cambridge: Cambridge University Press; 2007.
Merck & Co. Nasonex Product Monograph. http://www.merck.ca/assets/en/pdf/products/NASONEX-PM_E.pdf.
Sugano K, Okazaki A, Sugimoto S, Tavornvipas S, Omura A, Mano T. Solubility and dissolution profile assessment in drug discovery. Drug Metab Pharmacokinet. 2007;22(4):225–54.
Schipper NGM, Verhoef JC, Merkus FWHM. The nasal mucociliary clearance: relevance to nasal drug delivery. Pharm Res. 1991;8(7):807–14.
Keyhani K, Scherer PW, Mozell MM. A numerical model of nasal odorant transport for the analysis of human olfaction. J Theor Biol. 1997;186(3):279–301.
Lawson M, Craven B, Paterson E, Settles G. A computational study of odorant transport and deposition in the canine nasal cavity: implications for olfaction. Chem Senses. 2012;37(6):553–66.
U.S. Food and Drug Administration. Nasonex Product Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/20762s11lbl.pdf.
U.S. Pharmacopeia. Flunisolide Safety Data Sheet. http://www.usp.org/pdf/EN/referenceStandards/msds/1274505.pdf.
Azimi M, Longest PW, Hindle M. Towards Clinically Relevant In Vitro Testing of Locally Acting Nasal Spray Suspension Products. Respiratory Drug Delivery Europe. 2015
Gehr P, Schürch S, Berthiaume Y, HOF VI, Geiser M. Particle retention in airways by surfactant. J Aerosol Med. 1990;3(1):27–43.
Gehr P, Green F, Geiser M, Hof VI, Lee M, Schürch S. Airway surfactant, a primary defense barrier: mechanical and immunological aspects. J Aerosol Med. 1996;9(2):163–81.
Rygg A, Longest PW. Absorption and Clearance of Pharmaceutical Aerosols in the Human Nose: Development of a CFD Model. Journal of Aerosol Medicine and Pulmonary Drug Delivery. In review.
Longest PW, Vinchurkar S, Martonen T. Transport and deposition of respiratory aerosols in models of childhood asthma. J Aerosol Sci. 2006;37(10):1234–57.
Balashazy I, Hofmann W, Heistracher T. Computation of local enhancement factors for the quantification of particle deposition patterns in airway bifurcations. J Aerosol Sci. 1999;30(2):185–203.
Haghi M, Traini D, Young P. In vitro cell integrated impactor deposition methodology for the study of aerodynamically relevant size fractions from commercial pressurised metered dose inhalers. Pharm Res. 2014;31(7):1779–87.
Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61(2):158–71.
Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, et al. Drug Carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials. 2011;32(26):6285–90.
Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater. 2012;24(28):3887–94.
Sakagami M, Byron PR. Respirable Microspheres for inhalation. Clin Pharmacokinet. 2005;44(3):263–77.
Pereswetoff-Morath L. Microspheres as nasal drug delivery systems. Adv Drug Deliv Rev. 1998;29(1-2):185–94.
Illum L. Nasal clearance in health and disease. J Aerosol Med. 2006;19(1):92–9.
Bond S, Hardy J, Wilson C, editors. Deposition and Clearance of Nasal Sprays. The 2nd International Congress of Biopharmaceutics and Pharmacokinetics; 1984; Salamanca.
Rosen EJ, Calhoun KH. Alterations of nasal mucociliary clearance in association with HIV infection and the effect of Guaifenesin therapy. Laryngoscope. 2005;115(1):27–30.
Surico G, Muggeo P, Mappa L, Muggeo V, Conti V, Lucarelli A, et al. Impairment of nasal mucociliary clearance after radiotherapy for childhood head cancer. Head Neck. 2001;23(6):461–6.
Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163(4):983–8.
Illum L. Nasal drug delivery: New developments and strategies. Drug Discov Today. 2002;7(23):1184–9.
Patton JS, Brain JD, Davies La, Fiegel J, Gumbleton M, Kim K-J, et al. The Particle has Landed - Characterizing the Fate of Inhaled Pharmaceuticals. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2010;23, Supple:S71-S87.
Al-Ghananeem AM, Sandefer EP, Doll WJ, Page RC, Chang Y, Digenis GA. Gamma Scintigraphy for testing bioequivalence: a case study on Two cromolyn sodium nasal spray preparations. Int J Pharm. 2008;357(1):70–6.
Bur M, Huwer H, Muys L, Lehr C-M. Drug transport across pulmonary epithelial cell monolayers: effects of particle size, apical liquid volume, and deposition technique. J Aerosol Med Pulm Drug Deliv. 2010;23(3):119–27.
Henning A, Schneider M, Nafee N, Muijs L, Rytting E, Wang X, et al. Influence of particle size and material properties on mucociliary clearance from the airways. J Aerosol Med Pulm Drug Deliv. 2010;23(4):233–41.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18.
Hanson LR, Frey WH. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neuroscience. 2008;9 Suppl 3:S5.
Shanahan M. The spreading dynamics of a liquid drop on a viscoelastic solid. J Phys D Appl Phys. 1988;21(6):981.
Jacqmin D. Contact-line dynamics of a diffuse fluid interface. J Fluid Mech. 2000;402:57–88.
Dussan E. On the spreading of liquids on solid surfaces: static and dynamic contact lines. Annu Rev Fluid Mech. 1979;11(1):371–400.
Affrime MB, Cuss F, Padhi D, Wirth M, Pai S, Clement RP, et al. Bioavailability and metabolism of mometasone furoate following administration by metered‐dose and Dry‐powder inhalers in healthy human volunteers. J Clin Pharmacol. 2000;40(11):1227–36.
Daley-Yates P, Kunka R, Yin Y, Andrews S, Callejas S, Ng C. Bioavailability of fluticasone propionate and mometasone furoate aqueous nasal sprays. Eur J Clin Pharmacol. 2004;60(4):265–8.
ACKNOWLEDGMENTS AND DISCLOSURES
Dr. Geng Tian is gratefully acknowledged for his part in developing the NMT and nasal spray pump model during his time as a postdoc at Virginia Commonwealth University and Mandana Azimi for her critical review of the manuscript. This study was supported by Award U01 FD004570 and Contract HHSF223201310223C from the US FDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US FDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rygg, A., Hindle, M. & Longest, P.W. Absorption and Clearance of Pharmaceutical Aerosols in the Human Nose: Effects of Nasal Spray Suspension Particle Size and Properties. Pharm Res 33, 909–921 (2016). https://doi.org/10.1007/s11095-015-1837-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1837-5