Abstract
Purpose
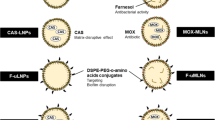
The aim of this study was to prepare wheat germ agglutinin (WGA)-modified liposomes encapsulating clarithromycin and to evaluate their in vitro and in vivo efficacy against Methicillin-resistant Staphylococcus aureus (MRSA).
Methods
Physicochemical parameters, minimum inhibitory concentrations, in vitro killing kinetic, cellular uptake, biofilm formation inhibition and pre-formed biofilm destruction, biodistribution, in vivo antibacterial efficacy against MRSA, and phagocytosis into macrophages for liposomes loading clarithromycin were determined.
Results
The minimum inhibitory concentration and the time–kill curve for WGA-modified liposomal clarithromycin were better than those of free and nonmodified liposomal clarithromycin. Flow cytometry analysis displayed that liposomes could deliver more Coumarin 6, a fluorescent probe, into bacteria because of the conjugation of WGA. Besides, WGA-modified liposomal clarithromycin inhibited formation of S. aureus (ATCC 29213) and MRSA biofiom, and prompted the biofilm disassembly at lower concentrations below MIC. Effective accumulation of liposomes was displayed in the enterocoelia of the mice because of WGA. The number of MRSA colony-forming units in the kidney and spleen in mice treated with WGA-modified liposomal clarithromycin was significantly lower than that treated with free and nonmodified clarithromycin (p < 0.05). Intracellular localization of MRSA occurred in a significantly higher proportion of macrophage exposed to WGA-modified liposomes compared to those exposed to nonmodified liposomes.
Conclusions
Liposome modified by WGA is a promising formulation for bacteria targeted delivery and immunity defensive system through macrophage improving uptake of bacteria, biodistribution, in vitro and in vivo antibacterial efficacy against MRSA.











Similar content being viewed by others
Abbreviations
- CLSM:
-
Confocal laser scanning microscope
- DC-Chol:
-
Cholesteryl 3β-N-di-methyl-amino-ethyl-carbamate hydrochloride
- DiR:
-
DiR iodide [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide]
- DMEM:
-
Dulbecco minimum essential medium
- DSPE-PEG:
-
DSPE-PEG2000
- Lip:
-
Clarithromycin loaded liposomes
- MIC:
-
Minimum inhibitory concentration
- MRSA:
-
Methicillin-resistant staphylococcus aureus
- NHS-PEG-DSPE:
-
N-hydroxysuccinimidyl-PEG2000-DSPE
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PEG-Lip:
-
Nonmodified liposomes
- S. aureus :
-
Staphylococcus aureus
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM:
-
Scanning Electron Microscopy
- SPC:
-
Soy phosphatidylcholine
- TSA:
-
Tryptic soy agar
- TSB:
-
Tryptic soy broth
- WGA:
-
Wheat germ agglutinin
- WGA-Lip:
-
WGA-modified liposomes
References
Walker B, Barrett S, Polasky S, Galaz V, Folke C, Engstrom G, et al. Environment. Looming global-scale failures and missing institutions. Science. 2009;325:1345–6.
Siegel RE. Emerging gram-negative antibiotic resistance: daunting challenges, declining sensitivities, and dire consequences. Respir Care. 2008;53:471–9.
Taubes G. The bacteria fight back. Science. 2008;321:356–61.
Teixeira PC, Leite GM, Domingues RJ, Silva J, Gibbs PA, Ferreira JP. Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. Int J Food Microbiol. 2007;118:15–9.
Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–28.
Goldsteinand IJ, Hayes CE. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340.
Sharma A, Sharma S, Khuller GK. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J Antimicrob Chemother. 2004;54:761–6.
Avni I, Arffa RC, Robin JB, Rao NA. Lectins for the identification of ocular bacterial pathogens. Metab Pediatr Syst Ophthalmol (New York, NY : 1985). 1987;10:45–7.
Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis : Off Publ Infect Dis Soc Am. 2004;39:971–9.
Ju RJ, Li XT, Shi JF, Li XY, Sun MG, Zeng F, et al. Liposomes, modified with PTD(HIV-1) peptide, containing epirubicin and celecoxib, to target vasculogenic mimicry channels in invasive breast cancer. Biomaterials. 2014;35:7610–21.
Yang K, Gitter B, Ruger R, Albrecht V, Wieland GD, Fahr A. Wheat germ agglutinin modified liposomes for the photodynamic inactivation of bacteria. Photochem Photobiol. 2012;88:548–56.
Cheng L, Huang FZ, Cheng LF, Zhu YQ, Hu Q, Li L, et al. GE11-modified liposomes for non-small cell lung cancer targeting: preparation, ex vitro and in vivo evaluation. Int J Nanomedicine. 2014;9:921–35.
C.a.L.S. Institute (2012) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Wayne, PA, USA. pp. Ninth Edition: Approved Standard M07-A09.
Sato T, Kawai Y, Matsuda H, Tateda K, Kimura S, Ishii Y, et al. In vitro and in vivo antibacterial activity of modithromycin against streptococci and Haemophilus influenzae. J Antimicrob Chemother. 2011;66:1547–54.
Zou Y, Lee Y, Huh J, Park JW. Synergistic effect of xylitol and ursolic acid combination on oral biofilms. Restor Dent Endod. 2014;39:288–95.
Weischenfeldtand J, Porse B. Bone Marrow-Derived Macrophages (BMM): isolation and applications. CSH Protocols. 2008;2008:pdb prot5080.
Gallily R, Douchan Z, Weiss DW. Potentiation of mouse peritoneal macrophage antibacterial functions by treatment of the donor animals with the methanol extraction residue fraction of tubercle bacilli. Infect Immun. 1977;18:405–11.
Lehr CM. Lectin-mediated drug delivery: the second generation of bioadhesives. J Controll Release : Off J Control Release Soc. 2000;65:19–29.
Valizadeh H, Mohammadi G, Ehyaei R, Milani M, Azhdarzadeh M, Zakeri-Milani P, et al. Antibacterial activity of clarithromycin loaded PLGA nanoparticles. Die Pharmazie. 2012;67:63–8.
Alhajlan M, Alhariri M, Omri A. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob Agents Chemother. 2013;57:2694–704.
Sanderson NM, Guo B, Jacob AE, Handley PS, Cunniffe JG, Jones MN. The interaction of cationic liposomes with the skin-associated bacterium Staphylococcus epidermidis: effects of ionic strength and temperature. Biochim Biophys Acta. 1996;1283:207–14.
Nagataand Y, Burger MM. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974;249:3116–22.
Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, Tulkens PM. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci. 2008;29:124–34.
Nishino T. An electron microscopic study of antagonism between cephalexin and erythromycin in Staphylococcus aureus. Jpn J Microbiol. 1975;19:53–63.
Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol. 2003;41:5–14.
Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Gotz F, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–76.
Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74:4849–55.
Kolodkin-Gal I, Cao S, Chai L, Bottcher T, Kolter R, Clardy J, et al. A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell. 2012;149:684–92.
Garg A, Tisdale AW, Haidari E, Kokkoli E. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. Int J Pharm. 2009;366:201–10.
Shen Y, Chen J, Liu Q, Feng C, Gao X, Wang L, et al. Effect of wheat germ agglutinin density on cellular uptake and toxicity of wheat germ agglutinin conjugated PEG-PLA nanoparticles in Calu-3 cells. Int J Pharm. 2011;413:184–93.
Glavas-Dodov M, Steffansen B, Crcarevska MS, Geskovski N, Dimchevska S, Kuzmanovska S, et al. Wheat germ agglutinin-functionalised crosslinked polyelectrolyte microparticles for local colon delivery of 5-FU: in vitro efficacy and in vivo gastrointestinal distribution. J Microencapsul. 2013;30:643–56.
Wang C, Ho PC, Lim LY. Wheat germ agglutinin-conjugated PLGA nanoparticles for enhanced intracellular delivery of paclitaxel to colon cancer cells. Int J Pharm. 2010;400:201–10.
Kesherwaniand V, Sodhi A. Differential activation of macrophages in vitro by lectin Concanavalin A, Phytohemagglutinin and Wheat germ agglutinin: production and regulation of nitric oxide. Nitric oxide : Biol Chem / Off J Nitric Oxide Soc. 2007;16:294–305.
Lotanand RM, Barzilai D. Effect of wheat germ agglutinin and concanavalin A on insulin binding and response by Madin-Darby canine kidney cells. Isr J Med Sci. 1990;26:5–11.
Rieger AM, Hall BE, Barreda DR. Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev Comp Immunol. 2010;34:1144–59.
Greenberg S, el Khoury J, di Virgilio F, Kaplan EM, Silverstein SC. Ca(2+)-independent F-actin assembly and disassembly during Fc receptor-mediated phagocytosis in mouse macrophages. J Cell Biol. 1991;113:757–67.
Aderemand A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623.
Corradin SB, Buchmuller-Rouiller Y, Mauel J. Phagocytosis enhances murine macrophage activation by interferon-gamma and tumor necrosis factor-alpha. Eur J Immunol. 1991;21:2553–8.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Natural Science Foundation of China (No. 81202488).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yansha Meng and Xucheng Hou contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3684 kb)
Rights and permissions
About this article
Cite this article
Meng, Y., Hou, X., Lei, J. et al. Multi-functional Liposomes Enhancing Target and Antibacterial Immunity for Antimicrobial and Anti-Biofilm Against Methicillin-Resistant Staphylococcus aureus . Pharm Res 33, 763–775 (2016). https://doi.org/10.1007/s11095-015-1825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1825-9