Abstract
Purpose
Characterize the contents of distal ileum and cecum in healthy adults under conditions simulating the bioavailability/bioequivelance studies of drug products in fasted and fed state.
Methods
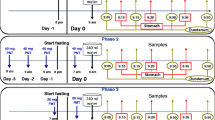
Twelve males participated in a two-phase crossover study. Phase I: subjects remained fasted overnight plus 4.5 h in the morning prior to colonoscopy. Phase II: subjects remained fasted overnight, consumed breakfast in the morning, and abstain from food until colonoscopy, 4.5 h after breakfast. Upon sampling, volume, pH and buffer capacity were measured; after ultracentrifugation, supernatant was physicochemically characterized and non-liquid particles diameter was measured.
Results
In distal ileum, pH is ~8.1 and size of non-liquid particles is ~200 μm, regardless of dosing conditions; in fed state, liquid fraction was lower whereas osmolality and carbohydrate content were higher. In cecum, the environment was similar with previously characterized environment in the ascending colon; in fasted state, size of non-liquid particles is smaller than in distal ileum (~70 μm). Fluid composition in distal ileum is different from cecum, especially in fasted state.
Conclusion
Differences in luminal environment between distal ileum and cecum may impact the performance of orally administered products which deliver drug during residence in lower intestine. Dosing conditions affect cecal environment more than in distal ileum.






Similar content being viewed by others
Abbreviations
- AA:
-
Acetic acid
- API:
-
Active pharmaceutical ingredient
- BA:
-
n-Butyric acid
- BA/BE:
-
Bioavailability/Bioequivalence
- BIS:
-
Bisacodyl
- C:
-
Cholic acid
- CA:
-
Caproic acid
- CDC:
-
Chenodeoxycholic acid
- CHO:
-
Cholesterol
- DC:
-
Deoxycholic acid
- DG:
-
Diglyceride
- FA:
-
Fatty acid
- GC:
-
Glycocholic acid
- GCDC:
-
Glycochenodeoxycholic acid
- GDC:
-
Glycodeoxycholic acid
- GI:
-
Gastrointestinal
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HIV:
-
Human Immunodeficiency Virus
- HPLC:
-
High performance liquid chromatography
- IBA:
-
Iso-butyric acid
- IVA:
-
Iso-valeric acid
- LC:
-
Lithocholic acid
- Lyso-PC:
-
Lyso-phosphatidylcholine
- MG:
-
Monoglyceride
- PA:
-
Propionic acid
- PC:
-
Phosphatidylcholine
- SCFA:
-
Short chain fatty acid
- SD:
-
Standard deviation
- TC:
-
Taurocholic acid
- TCDC:
-
Taurochenodeoxycholic acid
- TG:
-
Triglyceride
- UDC:
-
Ursodeoxycholic acid
- VA:
-
Valeric acid
References
Schiller C, Froehlich C-P, Giessmann T, Siegmund W, Moennikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by numeric resonance imaging. Aliment Pharmacol Ther. 2005;22:971–9.
Bergström CA, Holm R, Jørgensen SA, Andersson SB, Artursson P, Beato S, et al. Early pharmaceutical profiling to predict oral drug absorption: current status and unmet needs. Eur J Pharm Sci. 2014;57:173–99.
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76.
Diakidou A, Vertzoni M, Goumas K, Söderlind E, Abrahamsson B, Dressman J, et al. Characterization of the contents of ascending colon to which drugs are exposed after oral administration to healthy adults. Pharm Res. 2009;26:2141–51.
Vertzoni M, Goumas K, Söderlind E, Abrahamsson B, Dressman J, Poulou A, et al. Characterization of the ascending colon fluids in ulcerative colitis. Pharm Res. 2010;27:1620–6.
Vertzoni M, Diakidou A, Chatzilias M, Söderlind E, Abrahamsson B, Dressman JB, et al. Biorelevant media to simulate fluids in the ascending colon of humans and their usefulness in predicting intracolonic drug solubility. Pharm Res. 2010;27:2187–96.
Petrakis O, Vertzoni M, Angelou A, Kesisoglou F, Bentz K, Goumas K, et al. Identification of key factors affecting the oral absorption of salts of lipophilic weak acids: a case example. J Pharm Pharmacol. 2015;67(1):56–67.
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on the investigation of bioequivalence. January 2010. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 28 Dec 2014.
Kohn CW, Muir 3rd WW. Selected aspects of the clinical pharmacology of visceral analgesics and gut motility drugs in the horse. J Vet Intern Med. 1988;2:85–91.
Dhasmana KM, Banerjee AK, Erdmann W. Gastrointestinal transit following intrathecal or subcutaneous narcotic analgecics. Arch Int Pharmacodyn. 1987;286:152–61.
Sparkes AH, Papasouliotis K, Viner J, Cripps PJ, Gruffyd – Jones TJ. Assessment of orocaecal transit time in cats by the breath hydrogen method: the effects of sedation and a comparison of definitions. Res Vet Sci. 1996;60:243–6.
Washington N, Washington C, Wilson C. Physiological pharmaceutics: biological barriers to drug absorption. 2nd ed. London:Taylor and Francis; 2001.
Hernell O, Staggers JE, Carey MC. Physical-Chemical behaviour of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29:2041–56.
MacFarlane GT, MacFarlane S, Gibson GR. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb Ecol. 1998;35:180–7.
Apajalahti JH, Saerkilahti LK, Maeki BR, Heikkinen JP, Nurminen PH, Holben WE. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine-based analysis of community structure in the gastrointestinal tract of broiler chickens. Applied. Environ Microbiol. 1998;64:4084–8.
Vertzoni M, Carlsson A, Abrahamsson B, Goumas K, Reppas C. Degradation kinetics of metronidazole and olsalazine by bacteria in ascending colon and in feces of healthy adults. Int J Pharm. 2011;413:81–6.
Zhao G, Nyman M, Jinsson A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr. 2006;20:674–82.
Ladas SD, Isaacs PE, Murphy GM, Sladen GE. Fasting and postprandial ileal function in adapted ileostomates and normal subjects. Gut. 1986;27(8):906–12.
Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74(4):698–703.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger MB, Brewster M, et al. In vivo methods for drug absorption—Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:99–151.
Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment Pharmacol Ther. 1989;3(6):605–13.
Psachoulias D, Vertzoni M, Goumas K, Kalioras V, Beato S, Butler J, et al. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm Res. 2011;28:3145–58.
Vertzoni M, Markopoulos C, Symillides M, Goumas C, Imanidis G, Reppas C. Luminal lipid phases after administration of a triglyceride solution of danazol in the fed state and their contribution to the flux of danazol across caco-2 cell monolayers. Mol Pharm. 2012;9:1189–98.
Persson EM, Gustafsson AS, Carlsson AS, Nilsson RG, Knutson L, Forsell P, et al. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm Res. 2005;22(12):2141–51.
Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J, et al. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J Pharm Pharmacol. 2006;58:1079–89.
Fadda HM, Sousa T, Carlsson AS, Abrahamsson B, Williams JG, Kumar D, et al. Drug solubility in luminal fluids from different regions of the small and large intestine of humans. Mol Pharm. 2010;7(5):1527–32.
Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11(7):503–21.
Kumar V, Sinha AK, Makkar HP, de Boeck G, Becker K. Dietary roles of non-starch polysaccharides in human nutrition: a review. Crit Rev Food Sci Nutr. 2012;52(10):899–935.
Ladas SD, Isaacs PE, Murphy GM, Sladen GE. Comparison of the effects of medium and long chain triglyceride containing liquid meals on gall bladder and small intestinal function in normal man. Gut. 1984;25(4):405–11.
Davenport HW. Physiology of the digestive tract. An introductory text. 5th ed. Chicago: Year Book Medical Publishers, Inc; 1982.
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43.
ACKNOWLEDGMENTS AND DISCLOSURES
This work would not have been possible without the participation of reliable volunteers and authors would like to express their sincere appreciation.
Authors would like to thank Ms. Maria Koursari for her excellent technical assistance during colonoscopies.
Parts of the present work have been presented as an oral presentation at the “Progress within the IMI OrBiTo project—predictive tools for oral Biopharmaceutics” meeting (Academy of Pharmaceutical Sciences, Stevenage, UK, 13 May 2014) and as a poster at AAPS Annual Meeting, November 2–6, 2014, San Diego, California, USA.
This work was performed within the OrBiTo project (http://www.orbitoproject.eu) which is funded by the Innovative Medicines Initiative Joint Undertaking under Grant Agreement No 115369.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reppas, C., Karatza, E., Goumas, C. et al. Characterization of Contents of Distal Ileum and Cecum to Which Drugs/Drug Products are Exposed During Bioavailability/Bioequivalence Studies in Healthy Adults. Pharm Res 32, 3338–3349 (2015). https://doi.org/10.1007/s11095-015-1710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1710-6