Abstract
Purpose
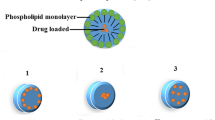
The debut study was aimed to develop Lactic acid (LA)-conjugated solid lipid nanoparticles (SLN-LA) bearing albendazole (ALB) and prednisolone (PRD) for effective management of neurocysticercosis (NCC).
Methods
LA was coupled to SLN by post-insertion technique. SLNs were characterized for particle size and size distribution, shape, and percent drug entrapment efficiency. In vitro drug release kinetics, fluorescence study and in vitro transendothelial transport, hematological studies and pharmacokinetic studies were carried out to predict the fullest drug delivery potential.
Results
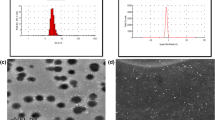
Spherical SLNs (~100 nm) with good drug entrapments (~64 and ~78% for ALB and PRD, respectively) showed in vitro initial fast release (i.e., 20–40% drugs release in 4 h) followed by sustained release for more than 48 h. Fluorescence study and in vitro transendothelial transport depicted selective brain uptake of SLN-LA compared to SLN attributed to carrier mediated transport via monocarboxylic acid transporters (MCT – 1/2/3). Pharmacokinetic parameters such as AUC0-t and AUMC0-t and Cllast showed good drugs withholding capacity of SLNs. Organ distribution studies reflected high accumulation of drugs (ALB, 7.6 ± 0.31%; PRD, 5.21 ± 0.24%) in the brain after 24 h in case of SLN-LA as compared to plain drugs solution. SLN-LA in hematological studies revealed insignificant toxicity to blood cells.
Conclusions
The overall study paved the potential advances in brain targeting with synergistic acting drugs for effective management of NCC.









Similar content being viewed by others
Abbreviations
- %EE:
-
Percent entrapment efficiency
- ACs:
-
Astrocyte cells
- ALB:
-
Albendazole
- BBB:
-
Blood brain barrier
- BCECs:
-
Brain capillary endothelial cells
- BCRP:
-
Breast cancer resistance protein
- CMT:
-
Carrier mediated transporters
- DDW:
-
Double distilled water
- EDC:
-
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- FITC:
-
Fluorescein isothiocyanate
- Hb:
-
Hemoglobin
- HSPC:
-
Hydrogenated soya phosphatidylcholine
- LA:
-
DL-lactic acid
- MCT:
-
Monocarboxylic acid transporters
- MRP:
-
Multidrug resistance proteins
- NCC:
-
Neurocysticercosis
- OLA:
-
OH protected LA
- P-gp:
-
P-glycoprotein
- PRD:
-
Prednisolone
- RBC:
-
Red blood corpuscles
- SA:
-
Stearylamine
- SD:
-
Standard deviation
- SLN-LA:
-
Lactic acid conjugated SLN loaded with ALB-PRD
- SLNs:
-
Solid lipid nanoparticles
- Sulfo-NHS:
-
N-Hydroxysulfosuccinimide
- WBC:
-
White blood corpuscles
- XRD:
-
X-ray diffraction
REFERENCES
Soteloand J, Del Brutto OH. Review of neurocysticercosis. Neurosurg Focus. 2002;12:1–7.
Garcia HH, Lescano AG, Lanchote VL, Pretell EJ, Gonzales I, Bustos JA, et al. Pharmacokinetics of combined treatment with praziquantel and albendazole in neurocysticercosis. Br J Clin Pharmacol. 2011;72:77–84.
Abbottand NJ, Romero IA. Transporting therapeutics across the blood–brain barrier. Mol Med Today. 1996;2:106–13.
Löscherand W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602.
Tsuji A. Small molecular drug transfer across the blood–brain barrier via carrier-mediated transport systems. NeuroRx. 2005;2:54–62.
Kim MH, Maeng HJ, Yu KH, Lee KR, Tsuruo T, Kim DD, et al. Evidence of carrier‐mediated transport in the penetration of donepezil into the rat brain. J Pharm Sci. 2010;99:1548–66.
Matthaiou DK, Panos G, Adamidi ES, Falagas ME. Albendazole versus praziquantel in the treatment of neurocysticercosis: a meta-analysis of comparative trials. PLoS Negl Trop Dis. 2008;2:e194.
Cruz I, Cruz M, Carrasco F, Horton J. Neurocysticercosis: optimal dose treatment with albendazole. J Neurol Sci. 1995;133:152–4.
Carpio A, Kelvin EA, Bagiella E, Leslie D, Leon P, Andrews H, et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008;79:1050–5.
Chattopadhyay N, Zastre J, Wong H-L, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25:2262–71.
Löscherand W, Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98.
Brasnjevic I, Steinbusch HW, Schmitz C, Martinez-Martinez P. Delivery of peptide and protein drugs over the blood–brain barrier. Prog Neurobiol. 2009;87:212–51.
Kharya P, Jain A, Gulbake A, Shilpi S, Jain A, Hurkat P, et al. Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting. J Nanoparticle Res. 2013;15:1–12.
Rai A, Jain A, Jain A, Jain A, Pandey V, Chashoo G, et al. Targeted SLNs for management of HIV-1 associated dementia. Drug Dev Ind Pharm. 2014;1–7.
Redzic Z. Molecular biology of the blood–brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:1–25.
Mamta Bishnoi AJ, Pooja Hurkat, Jain SK. Aceclofenac-loaded chondroitin sulfate conjugated SLNs for effective management of osteoarthritis. J Drug Target. 2014;1–8.
MuÈller RH, MaÈder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77.
Müller R, Hildebrand G, Nitzsche R, Paulke B-R. Zetapotential und Partikelladung in der Laborpraxis. PAPERBACK APV. 1996;37.
Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release. 2012;163:34–45.
Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127:97–109.
Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59:454–77.
Jain A, Jain SK. Brain Targeting Using Surface Functionalized Nanocarriers in human solid tumors. In: Singh B, Jain NK, Katare OP, editors. Drug nanocarriers. Houston: Series Nanobiomedicine Studium Press; 2014. p. 203–55.
Kennedyand KM, Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–48.
Schubertand M, Müller-Goymann C. Solvent injection as a new approach for manufacturing lipid nanoparticles–evaluation of the method and process parameters. Eur J Pharm Biopharm. 2003;55:125–31.
Kocienski PJ. Protecting groups. Thieme. 2005.
Greeneand TW, Wuts PG. Protection for the Hydroxyl Group, Including 1, 2‐and 1, 3‐Diols. 3rd ed. Protective Groups in Organic Synthesis; 1999 pp. 17–245.
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20.
Jain A, Gulbake A, Jain A, Shilpi S, Hurkat P, Jain SK. Dual drug delivery using “smart” liposomes for triggered release of anticancer agents. J Nanoparticle Res. 2013;15:1–12.
Truong Cong T, Faivre V, Nguyen TT, Heras H, Pirot F, Walchshofer N, et al. Study on the hydatid cyst membrane: permeation of model molecules and interactions with drug-loaded nanoparticles. Int J Pharm. 2008;353:223–32.
Souto EB, Fangueiro JF, Müller RH. Solid Lipid Nanoparticles (SLN™). Fundamentals of pharmaceutical nanoscience, Springer: 2013, pp. 91–116.
Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine Nanotechnol Biol Med. 2009;5:184–91.
Bishnoi M, Jain A, Hurkat P, Jain SK. Aceclofenac-loaded chondroitin sulfate conjugated SLNs for effective management of osteoarthritis. J Drug Target. 2014;1–8.
Venkateswarluand V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004;95:627–38.
Manjunathand K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of nitrendipine solid lipid nanoparticles after intravenous and intraduodenal administration. J Drug Target. 2006;14:632–45.
Göppertand TM, Müller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13:179–87.
Kido Y, Tamai I, Okamoto M, Suzuki F, Tsuji A. Functional clarification of MCT1-mediated transport of monocarboxylic acids at the blood–brain barrier using in vitro cultured cells and in vivo BUI studies. Pharm Res. 2000;17:55–62.
Tamai I, Takanaga H, Ogihara T, Higashida H, Maeda H, Sai Y, et al. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem Biophys Res Commun. 1995;214:482–9.
Hertzand L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res. 2005;79:11–8.
Agarwal A, Agrawal H, Tiwari S, Jain S, Agrawal GP. Cationic ligand appended nanoconstructs: a prospective strategy for brain targeting. Int J Pharm. 2011;421:189–201.
ACKNOWLEDGMENTS AND DISCLOSURES
Authors are thankful to Mino Pharma and Sain Medicaments (Hyderabad, India) and Synmedic Laboratories, Faridabad (Haryana, India) for providing gift samples of drugs. We wish to acknowledge the support of Lipoid (Ludwigshafen Am Rhein, Germany) for rendering gift samples of lipids. The authors are also thankful to AIIMS, New Delhi for carrying out TEM and SEM studies. Authors namely Mr. Ankit Jain and Ms. Pooja Hurkat are highly obliged to Council of Scientific and Industrial Research (New Delhi, India) for rendering Senior Research Fellowship (SRF). The authors declare no competing financial interest and they alone are responsible for the content and writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devi, R., Jain, A., Hurkat, P. et al. Dual Drug Delivery Using Lactic Acid Conjugated SLN for Effective Management of Neurocysticercosis. Pharm Res 32, 3137–3148 (2015). https://doi.org/10.1007/s11095-015-1677-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1677-3