Abstract
Purpose
Neutropenia is a major dose-limiting side effect of chemotherapy and is closely related to febrile neutropenia which mainly occurs during the first cycle. Our objectives were to establish model-based decision rules from early absolute neutrophil counts (ANC) to anticipate prolonged high grade neutropenia at cycle 1 and to prevent it through delayed granulocyte colony stimulating factor (G-CSF) administration in carboplatin-treated patients.
Methods
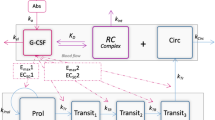
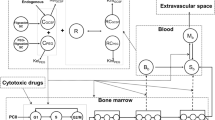
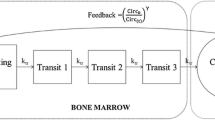
The decision rules were built from Monte Carlo simulations performed with a previously published semi-mechanistic model describing ANC time-course in carboplatin-treated patients with or without concomitant G-CSF therapy.
Results
ANC measured at day 0 (D0, baseline), D4 and D5 were good predictors of prolonged high grade neutropenia at cycle 1. Pegfilgrastim administration on D5 was as effective as the conventional pegfilgrastim administration on D1 but none avoided prolonged high grade neutropenia in all patients. Additional decision rules were thus derived, using the same ANC combination, to identify patients for whom G-CSF was beneficial. All decision rules showed good performances (sensitivity/specificity).
Conclusion
We propose an innovative approach to guide oncologist in their clinical practice. The next step is to perform prospective studies to implement, validate and possibly refine the proposed decision rules.




Similar content being viewed by others
Abbreviations
- ANC:
-
Absolute neutrophils counts
- G-CSF:
-
Granulocyte colony-stimulating factor
- ROC:
-
Receiver-operating characteristic
References
Cameron D. Management of chemotherapy-associated febrile neutropenia. Br J Cancer. 2009;101 Suppl 1:S18–22.
Lyman GH, Kuderer NM. Epidemiology of febrile neutropenia. Support Cancer Ther. 2003;1:23–35.
Gascon P, Aapro M, Ludwig H, Rosencher N, Turner M, Song M, et al. Background and methodology of MONITOR-GCSF, a pharmaco-epidemiological study of the multi-level determinants, predictors, and clinical outcomes of febrile neutropenia prophylaxis with biosimilar granulocyte-colony stimulating factor filgrastim. Crit Rev Oncol Hematol. 2011;77:184–97.
Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–40.
Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228–37.
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35.
Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–31.
Kelly S, Wheatley D. Prevention of febrile neutropenia: use of granulocyte colony-stimulating factors. Br J Cancer. 2009;101 Suppl 1:S6–10.
Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer. 2010;18:529–41.
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404.
Segal BH, Freifeld AG, Baden LR, Brown AE, Casper C, Dubberke E, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2008;6:122–74.
Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci U S A. 1989;86:9499–503.
Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–40.
Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–53.
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205.
Friberg LE, Karlsson MO. Mechanistic models for myelosuppression. Invest New Drugs. 2003;21:183–94.
Shochat E, Rom-Kedar V, Segel LA. G-CSF control of neutrophils dynamics in the blood. Bull Math Biol. 2007;69:2299–338.
Testart-Paillet D, Girard P, You B, Freyer G, Pobel C, Tranchand B. Contribution of modelling chemotherapy-induced hematological toxicity for clinical practice. Crit Rev Oncol Hematol. 2007;63:1–11.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20:4713–21.
Hansson EK, Friberg LE. The shape of the myelosuppression time profile is related to the probability of developing neutropenic fever in patients with docetaxel-induced grade IV neutropenia. Cancer Chemother Pharmacol. 2012;69:881–90.
Wallin JE, Friberg LE, Karlsson MO. A tool for neutrophil guided dose adaptation in chemotherapy. Comput Methods Programs Biomed. 2009;93:283–91.
Wallin JE, Friberg LE, Karlsson MO. Model-based neutrophil-guided dose adaptation in chemotherapy: evaluation of predicted outcome with different types and amounts of information. Basic Clin Pharmacol Toxicol. 2010;106:234–42.
Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003;98:2402–9.
Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6:109–18.
Pastor ML, Laffont CM, Gladieff L, Schmitt A, Chatelut E, Concordet D. Model-based approach to describe G-CSF effects in carboplatin-treated cancer patients. Pharm Res. 2013;30:2795–807.
Yang BB, Lum PK, Hayashi MM, Roskos LK. Polyethylene glycol modification of filgrastim results in decreased renal clearance of the protein in rats. J Pharm Sci. 2004;93:1367–73.
Roskos LK, Lum P, Lockbaum P, Schwab G, Yang BB. Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol. 2006;46:747–57.
Scholz M, Schirm S, Wetzler M, Engel C, Loeffler M. Pharmacokinetic and -dynamic modelling of G-CSF derivatives in humans. Theor Biol Med Model. 2012;9:32.
Krzyzanski W, Wiczling P, Lowe P, Pigeolet E, Fink M, Berghout A, et al. Population modeling of filgrastim PK-PD in healthy adults following intravenous and subcutaneous administrations. J Clin Pharmacol. 2010;50:101S–12.
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32.
Crawford J, Caserta C, Roila F. Hematopoietic growth factors: ESMO recommendations for the applications. Ann Oncol. 2009;20 Suppl 4:162–5.
Morstyn G, Campbell L, Lieschke G, Layton JE, Maher D, O’Connor M, et al. Treatment of chemotherapy-induced neutropenia by subcutaneously administered granulocyte colony-stimulating factor with optimization of dose and duration of therapy. J Clin Oncol. 1989;7:1554–62.
Meisenberg BR, Davis TA, Melaragno AJ, Stead R, Monroy RL. A comparison of therapeutic schedules for administering granulocyte colony-stimulating factor to nonhuman primates after high-dose chemotherapy. Blood. 1992;79:2267–72.
Yankelevich M, Goodell MA, Kaplan J. Efficacy of delayed administration of post-chemotherapy granulocyte colony-stimulating factor: evidence from murine studies of bone marrow cell kinetics. Exp Hematol. 2008;36:9–16.
Vainas O, Ariad S, Amir O, Mermershtain W, Vainstein V, Kleiman M, et al. Personalising docetaxel and G-CSF schedules in cancer patients by a clinically validated computational model. Br J Cancer. 2012;107:814–22.
Tan H, Tomic K, Hurley D, Daniel G, Barron R, Malin J. Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin. 2011;27:79–86.
Whyte S, Cooper KL, Stevenson MD, Madan J, Akehurst R. Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health. 2011;14:465–74.
Weycker D, Malin J, Barron R, Edelsberg J, Kartashov A, Oster G. Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy. Am J Clin Oncol. 2012;35:267–74.
Barbolosi D, Iliadis A. Optimizing drug regimens in cancer chemotherapy: a simulation study using a PK-PD model. Comput Biol Med. 2001;31:157–72.
de Jonge ME, Huitema AD, Schellens JH, Rodenhuis S, Beijnen JH. Individualised cancer chemotherapy: strategies and performance of prospective studies on therapeutic drug monitoring with dose adaptation: a review. Clin Pharmacokinet. 2005;44:147–73.
Evene E, Chatelut E, Tranchand B, Canal P, Lochon I, Iliadis A. and Ardiet CJ [Bayesian estimation of pharmacokinetic parameters of etoposide]. Bull Cancer. 1997;84:699–703.
Rousseau A, Marquet P, Debord J, Sabot C, Lachatre G. Adaptive control methods for the dose individualisation of anticancer agents. Clin Pharmacokinet. 2000;38:315–53.
Tranchand B, Amsellem C, Chatelut E, Freyer G, Iliadis A, Ligneau B, et al. A limited-sampling strategy for estimation of etoposide pharmacokinetics in cancer patients. Cancer Chemother Pharmacol. 1999;43:316–22.
ACKNOWLEDGMENTS AND DISCLOSURES
We have no competing interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 115 kb)
Rights and permissions
About this article
Cite this article
Pastor, M.L., Laffont, C.M., Gladieff, L. et al. Model-Based Approach to Early Predict Prolonged High Grade Neutropenia in Carboplatin-Treated Patients and Guide G-CSF Prophylactic Treatment. Pharm Res 32, 654–664 (2015). https://doi.org/10.1007/s11095-014-1493-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1493-1