Abstract
Purpose
It is well known that primary emulsion (W1/O) preparation process (by ultrasonication or homogenization) plays an important role in the properties of drug-loaded microspheres, such as encapsulation efficiency, release behavior and pharmacodynamics. However, its involved mechanism has not been intensively and systematically studied, partly because that broad size distribution of the resultant particles prepared by conventional preparation can greatly disturb the analysis and reliability of the results. Here, we focused on the relevant studies.
Methods
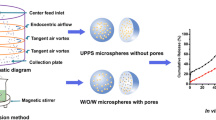
In order to eliminate the disturbance caused by broad size distribution, uniform-sized exenatide-loaded poly(DL-lactic-co-glycolic acid) (PLGA) microspheres were prepared by Shirasu Porous Glass (SPG) premix membrane emulsification. The properties of microspheres whose W1/O was formed by ultrasonication (UMS) and homogenization (HMS) were compared including in vitro release, pharmacology and so forth.
Results
HMS exhibited fast release rate and hyperglycemic efficacy within first 14 days, but declined afterwards. Comparatively, UMS showed slower polymer degradation, more acidic microclimate pH (μpH) in vitro, and stable drug release with sustained efficacy during 1 month in vivo.
Conclusions
HMS was desirable for the 2-week-sustained release in vivo, while UMS was more appropriate for the longer time release (about 1 month). These comparative researches can provide guidance for emulsion-microsphere preparation routs in pharmaceutics.







Similar content being viewed by others
References
Mao SR, Xu J, Cai CF, et al. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007;334(1–2):137–48.
Sah HK, Toddywala R, Chien YW. Biodegradable microcapsules prepared by a W/O/W technique —effects of shear force to make a primary W/O emulsion on their morphology and protein release. J Microencapsul. 1995;12(1):59–69.
Yan CH, Resau JH, Hewetson J, et al. Characterization and morphological analysis of protein-loaded poly(lactide-co-glycolide) microparticles prepared by water-in oil-in-water emulsion technique. J Control Release. 1994;32(3):231–41.
Blanco D, Alonso MJ. Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur J Pharm Biopharm. 1998;45(3):285–94.
van de Weert M, Hoechstetter J, Hennink WE, et al. The effect of a water/organic solvent interface on the structural stability of lysozyme. J Control Release. 2000;68(3):351–9.
Morlock M, Koll H, Winter G, et al. Microencapsulation of rh-erythropoietin, using biodegradable poly(D, L-lactide-co-glycolide): protein stability and the effects of stabilizing excipients. Eur J Pharm Biopharm. 1997;43(1):29–36.
Zambaux MF, Bonneaux F, Gref R, et al. Preparation and characterization of protein C-loaded PLA nanoparticles. J Control Release. 1999;60(2–3):179–88.
Cai CF, Mao SR, Germershaus O, et al. Influence of morphology and drug distribution on the release process of FITC-dextran-loaded microspheres prepared with different types of PLGA. J Microencapsul. 2009;26(4):334–45.
Wang LY, Gu YH, Zhou QZ, et al. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf, B. 2006;50(2):126–35.
Liu R, Ma G, Meng F, et al. Preparation of uniform-sized PLA microcapsules by combining Shirasu Porous Glass membrane emulsification technique and multiple emulsion-solvent evaporation method. J Control Release. 2005;103(1):31–43.
Liu R, Ma GH, Wan YH, et al. Influence of process parameters on the size distribution of PLA microcapsules prepared by combining membrane emulsification technique and double emulsion-solvent evaporation method. Colloids Surf, B. 2005;45(3–4):144–53.
Liu R, Huang SS, Wan YH, et al. Preparation of insulin-loaded PLA/PLGA microcapsules by a novel membrane emulsification method and its release in vitro. Colloids Surf, B. 2006;51(1):30–8.
Wei Q, Wei W, Tian R, et al. Preparation of uniform-sized PELA microspheres with high encapsulation efficiency of antigen by premix membrane emulsification. J Colloid Interf Sci. 2008;323(2):267–73.
Ahn JH, Park EJ, Lee HS, et al. Reversible blocking of amino groups of octreotide for the inhibition of formation of acylated peptide impurities in poly(lactide-co-glycolide) delivery systems. Aaps Pharmscitech. 2011;12(4):1220–6.
Qi F, Wu J, Fan Q, et al. Preparation of uniform-sized exenatide-loaded PLGA microspheres as long-effective release system with high encapsulation efficiency and bio-stability. Colloids Surf, B. 2013;112:492–8.
Wei Y, Wang Y, Wang L, et al. Fabrication strategy for amphiphilic microcapsules with narrow size distribution by premix membrane emulsification. Colloids Surf, B. 2011;87(2):399–408.
Priego-Capote F, de Castro L. Analytical uses of ultrasound - I. Sample preparation. Trac-Trend Anal Chem. 2004;23(9):644–53.
Wei GB, Pettway GJ, McCauley LK, et al. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25(2):345–52.
Yang YY, Chung TS, Bai XL, et al. Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method. Chem Eng Sci. 2000;55(12):2223–36.
Freiberg S, Zhu X. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282(1–2):1–18.
Yushu H, Venkatraman S. The effect of process variables on the morphology and release characteristics of protein-loaded PLGA particles. J Appl Polym Sci. 2006;101(5):3053–61.
Ding AG, Schwendeman SP. Acidic microclimate pH distribution in PLGA microspheres monitored by confocal laser scanning microscopy. Pharm Res. 2008;25(9):2041–52.
Liu YJ, Schwendeman SP. Mapping microclimate pH distribution inside protein-Encapsulated PLGA microspheres using confocal laser scanning microscopy. Mol Pharmaceut. 2012;9(5):1342–50.
Li L, Schwendeman SP. Mapping neutral microclimate pH in PLGA microspheres. J Control Release. 2005;101(1–3):163–73.
Venier-Julienne MC, Giteau A, Aubert-Pouessel A, et al. How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharm. 2008;350(1–2):14–26.
Liang R, Li X, Shi Y, et al. Effect of water on exenatide acylation in poly(lactide-co-glycolide) microspheres. Int J Pharm. 2013;454(1):344–53.
Schwendeman SP, Sophocleous AM, Zhang Y. A new class of inhibitors of peptide sorption and acylation in PLGA. J Control Release. 2009;137(3–4):179–84.
Tracy MA, Ward KL, Firouzabadian L, et al. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;20(11):1057–62.
Acknowledgments and Disclosures
Feng Qi and Jie Wu contributed equally to this work. We thank the Joint NSFC/RGC grant (21161160555) for the financial support provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, F., Wu, J., Hao, D. et al. Comparative Studies on the Influences of Primary Emulsion Preparation on Properties of Uniform-Sized Exenatide-Loaded PLGA Microspheres. Pharm Res 31, 1566–1574 (2014). https://doi.org/10.1007/s11095-013-1262-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1262-6