ABSTRACT
Purpose
To investigate mechanisms governing the stabilization and destabilization of immunoglobulin (IgG1) by arginine (Arg).
Methods
The effects of Arg on the aggregation/degradation, thermodynamic stability, hydrophobicity, and aromatic residues of IgG1 were respectively investigated by size-exclusion chromatography, differential scanning calorimetry, probe fluorescence, and intrinsic fluorescence.
Results
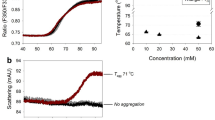
Arg monohydrochloride (Arg–HCl) suppressed IgG1 aggregation at near-neutral pH, but facilitated aggregation and degradation at acidic pH or at high storage temperature. Equimolar mixtures of Arg and aspartic acid (Asp) or glutamic acid (Glu) suppressed aggregation without facilitating degradation even at high temperature. Arg–HCl decreased the thermodynamic stability of IgG1 by enthalpic loss, which was counteracted by using Asp or Glu as a counterion for Arg. The suppression of aggregation by Arg–HCl was well correlated with the decrease in hydrophobicity of IgG1. The intrinsic fluorescence of IgG1 was unaffected by Arg–HCl.
Conclusions
Suppression of IgG1 aggregation can be attributed to the interaction between Arg and hydrophobic residues; on the other hand, facilitation of aggregation and degradation is presumably due to the interaction between Arg and some acidic residues, which could be competitively inhibited by simultaneously adding either Asp or Glu.









Similar content being viewed by others
Abbreviations
- Arg–Asp:
-
Arginine–aspartic acid mixture
- Arg–Glu:
-
Arginine–glutamic acid mixture
- Arg–HCl:
-
Arginine monohydrochloride
- Bis-ANS:
-
4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid dipotassium salt
- C p :
-
Molar heat capacity at constant pressure
- DSC:
-
Differential scanning calorimetry
- SEC:
-
Size-exclusion chromatography
REFERENCES
Wang W, Singh SK, Li N, Toler MR, King KR, Nema S. Immunogenicity of protein aggregates—concerns and realities. Int J Pharm. 2012;431:1–11.
Buchner J, Rudolph R. Renaturation, purification and characterization of recombinant Fab-fragments produced in Escherichia coli. Biotechnology (N Y). 1991;9:157–62.
Arora D, Khanna N. Method for increasing the yield of properly folded recombinant human gamma interferon from inclusion bodies. J Biotechnol. 1996;52:127–33.
Tsumoto K, Umetsu M, Kumagai I, Ejima D, Arakawa T. Solubilization of active green fluorescent protein from insoluble particles by guanidine and arginine. Biochem Biophys Res Commun. 2003;312:1383–6.
Umetsu M, Tsumoto K, Nitta S, Adschiri T, Ejima D, Arakawa T, et al. Nondenaturing solubilization of beta2 microglobulin from inclusion bodies by L-arginine. Biochem Biophys Res Commun. 2005;328:189–97.
Arakawa T, Philo JS, Tsumoto K, Yumioka R, Ejima D. Elution of antibodies from a Protein-A column by aqueous arginine solutions. Protein Expr Purif. 2004;36:244–8.
Ejima D, Yumioka R, Tsumoto K, Arakawa T. Effective elution of antibodies by arginine and arginine derivatives in affinity chromatography. Anal Biochem. 2005;345:250–7.
Ejima D, Yumioka R, Arakawa T, Tsumoto K. Arginine as an effective additive in gel permeation chromatography. J Chromatogr A. 2005;1094:49–55.
Hautbergue GM, Golovanov AP. Increasing the sensitivity of cryoprobe protein NMR experiments by using the sole low-conductivity arginine glutamate salt. J Magn Reson. 2008;191:335–9.
Placzek WJ, Almeida MS, Wüthrich K. NMR structure and functional characterization of a human cancer-related nucleoside triphosphatase. J Mol Biol. 2007;367:788–801.
Song J, Zhao KQ, Newman CL, Vinarov DA, Markley JL. Solution structure of human sorting nexin 22. Protein Sci. 2007;16:807–14.
Tsumoto K, Umetsu M, Kumagai I, Ejima D, Philo JS, Arakawa T. Role of arginine in protein refolding, solubilization, and purification. Biotechnol Prog. 2004;20:1301–8.
Arakawa T, Ejima D, Tsumoto K, Obeyama N, Tanaka Y, Kita Y, et al. Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. Biophys Chem. 2007;127:1–8.
Ghosh R, Sharma S, Chattopadhyay K. Effect of arginine on protein aggregation studied by fluorescence correlation spectroscopy and other biophysical methods. Biochemistry. 2009;48:1135–43.
Ito L, Shiraki K, Matsuura T, Okumura M, Hasegawa K, Baba S, et al. High-resolution X-ray analysis reveals binding of arginine to aromatic residues of lysozyme surface: implication of suppression of protein aggregation by arginine. Protein Eng Des Sel. 2011;24:269–74.
Das U, Hariprasad G, Ethayathulla AS, Manral P, Das TK, Pasha S, et al. Inhibition of protein aggregation: supramolecular assemblies of arginine hold the key. PLoS One. 2007;2:e1176.
Shukla D, Trout BL. Interaction of arginine with proteins and the mechanism by which it inhibits aggregation. J Phys Chem B. 2010;114:13426–38.
Shah D, Shaikh RA, Peng X, Rajagopalan R. Effects of arginine on heat-induced aggregation of concentrated protein solutions. Biotechnol Prog. 2011;27:513–20.
Kameoka D, Ueda T, Imoto T. Effect of the conformational stability of the CH2 domain on the aggregation and peptide cleavage of a humanized IgG. Appl Biochem Biotechnol. 2011;164:642–54.
Kameoka D, Masuzaki E, Ueda T, Imoto T. Effect of buffer species on the unfolding and the aggregation of humanized IgG. J Biochem. 2007;142:383–91.
Tischenko VM, Zav’yalov VP, Medgyesi GA, Potekhin SA, Privalov PL. A thermodynamic study of cooperative structures in rabbit immunoglobulin G. Eur J Biochem. 1982;126:517–21.
Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G. Potential inaccurate quantitation and sizing of protein aggregates by size exclusion chromatography: essential need to use orthogonal methods to assure the quality of therapeutic protein products. J Pharm Sci. 2010;99:2200–8.
Lilyestrom WG, Shire SJ, Scherer TM. Influence of the cosolute environment on IgG solution structure analyzed by small-angle X-ray scattering. J Phys Chem B. 2012;116:9611–8.
Nakakido M, Tanaka Y, Mitsuhori M, Kudou M, Ejima D, Arakawa T, et al. Structure-based analysis reveals hydration changes induced by arginine hydrochloride. Biophys Chem. 2008;137:105–9.
Golovanov AP, Hautbergue GM, Wilson SA, Lian LY. A simple method for improving protein solubility and long-term stability. J Am Chem Soc. 2004;126:8933–9.
Shukla D, Trout BL. Understanding the synergistic effect of arginine and glutamic acid mixtures on protein solubility. J Phys Chem B. 2011;115:11831–9.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Dr. Kohei Tsumoto (Medical Proteomics Laboratory, Institute of Medical Science, The University of Tokyo) for thoughtful discussions. The authors also thank many individuals of our departments for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, M., Kameoka, D., Torizawa, T. et al. Thermodynamic and Fluorescence Analyses to Determine Mechanisms of IgG1 Stabilization and Destabilization by Arginine. Pharm Res 31, 992–1001 (2014). https://doi.org/10.1007/s11095-013-1221-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1221-2