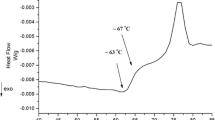
Abstract
Purpose
Predicting thermal protein stability is of major interest in the development of protein-based biopharmaceuticals. Therefore, this study provides a predictive tool for determining transition enthalpies, which can be used for ranking different proteins according to their thermal stability.
Methods
Unfolding and aggregation profiles of eight different therapeutic monoclonal antibodies (mAbs) of type G, isotype 1 were investigated. The unfolding profiles were determined by intrinsic fluorescence (IF) spectroscopy and differential scanning calorimetry (DSC). A three-state unfolding fitting model was used to determine thermodynamic parameters for macromolecular multi-domain mAbs in IF experiments, like the van’t Hoff enthalpy change (∆Hvh) and the entropy change (∆S) of the unfolding event. The derived values were compared to thermodynamic parameters obtained directly by calorimetry. Moreover, differences in the Fab enthalpies were used to predict aggregation behavior and protein thermal stabilities. To do so, the liquid-formulated mAbs were investigated exemplarily by size exclusion chromatography (SEC) after accelerated thermal-induced stress conditions.
Results
Comparing the thermodynamic parameters derived from IF spectroscopy and DSC resulted in similar values. Data generated by thermal-induced stress at 40°C show similar stability ranking as postulated through the Fab enthalpies for mAbs in two different formulations, while at 25°C a meaningful ranking is not possible, because distinct differences in the thermal stability cannot be observed. The additional consideration of Fab enthalpies to predict the 40 °C SEC ranking seems to be more reliable compared to the use of exclusively the melting temperatures or aggregation onset temperatures and times.
Conclusion
We show that thermodynamic profiling can help predicting unfolding and aggregation properties of therapeutic mAbs at 40°C. Therefore, analyzing thermodynamic unfolding parameters is a useful and supportive tool discriminating thermal stability profiles of mAbs for further pharmaceutical development and clinical studies.





Similar content being viewed by others
Abbreviations
- ∆cp :
-
heat capacity change
- ∆G:
-
Gibbs free energy
- ∆Hcal :
-
calorimetric enthalpy
- ∆Hvh :
-
van’t Hoff enthalpy
- ∆S:
-
entropy
- CH-domain:
-
constant antibody domain of the heavy chain
- CHO:
-
Chinese Hamster Ovary
- cp,Tm :
-
heat capacity of the melting temperature
- c.u.:
-
cooperative unit
- DSC:
-
differential scanning calorimetry
- F:
-
folded state
- f:
-
state fraction
- Fab:
-
fragment antigen binding
- Fc:
-
fragment crystallizable
- FE330 :
-
fluorescence emission at 330 nm
- FE350 :
-
fluorescence emission at 350 nm
- HP/UP-SEC:
-
high performance / ultra performance size exclusion chromatography
- I:
-
intermediate state
- IF:
-
intrinsic fluorescence spectroscopy
- IgG1:
-
immunoglobulin type G isotype 1
- K:
-
equilibrium constant
- LENP:
-
Lumry-Eyring nucleated polymerization
- mAb:
-
monoclonal antibody
- mAU:
-
milli arbitrary units
- MWCO:
-
molecular weight cut off
- n:
-
reaction order
- R:
-
gas constant
- S:
-
slope
- T:
-
temperature
- Tagg :
-
aggregation onset temperature
- tagg :
-
aggregation onset time
- Tm :
-
melting temperature
- U:
-
unfolded state
- y:
-
signals of the IF thermograms
- Y:
-
single state signals
References
Roberts CJ, Das TK, Sahin E. Predicting solution aggregation rates for therapeutic proteins: approaches and challenges. Int J Pharm. 2011;418(2):318–33.
Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289(1–2):1–30.
Wang W, Nema S, Teagarden D. Protein aggregation--pathways and influencing factors. Int J Pharm. 2010;390(2):89–99.
den Engelsman J, Garidel P, Smulders R, Koll H, Smith B, Bassarab S, et al. Strategies for the assessment of protein aggregates in pharmaceutical biotech product development. Pharm Res. 2011;28(4):920–33.
Dill KA. Dominant forces in protein folding. Biochemistry. 1990;19(31):7133–55.
Meuzelaar H, Vreede J, Woutersen S. Influence of Glu/Arg, asp/Arg, and Glu/Lys salt bridges on α-helical stability and folding kinetics. Biophys J. 2016;110(11):2328–41.
Kubelka J, Henry ER, Cellmer T, Hofrichter J, Eaton WA. Chemical, physical, and theoretical kinetics of an ultrafast folding protein. Proc Natl Acad Sci. 2008;105(48):18655–62.
Boehm K, Guddorf J, Albers A, Kamiyama T, Fetzner S, Hinz HJ. Thermodynamic analysis of denaturant-induced unfolding of HodC69S protein supports a three-state mechanism. Biochemistry. 2008;47(27):7116–26.
Davis CM, Dyer RB. The role of electrostatic interactions in folding of β-proteins. J Am Chem Soc. 2016;138(4):1456–64.
Naganathan AN, Doshi U, Muñoz V. Protein folding kinetics: barrier effects in chemical and thermal denaturation experiments. J Am Chem Soc. 2007;129(17):5673–82.
Beermann B, Guddorf J, Boehm K, Albers A, Kolkenbrock S, Fetzner S, et al. Stability, unfolding, and structural changes of cofactor-free 1H-3-Hydroxy-4-oxoquinaldine 2,4-Dioxygenase. Biochemistry. 2007;46(14):4241–9.
Zimm BH, Bragg JK. Theory of the phase transition between helix and random coil in polypeptide chains. J Chem Phys. 1959;31(2):526–35.
Seelig J, Schönfeld H-J. Thermal protein unfolding by differential scanning calorimetry and circular dichroism spectroscopy two-state model versus sequential unfolding. Q Rev Biophys. 2016;49.
Li-Blatter X, Seelig J. Thermal and chemical unfolding of lysozyme. Multistate Zimm–Bragg theory versus two-state model. J Phys Chem B. 2019;123(48):10181–91.
Garidel P, Eiperle A, Blech M, Seelig J. Thermal and chemical unfolding of a monoclonal IgG1 antibody: application of the multi-state Zimm-Bragg theory. Biophys J. 2020;118:4067–1075.
Blaffert J, Haeri HH, Blech M, Hinderberger D, Garidel P. Spectroscopic methods for assessing the molecular origins of macroscopic solution properties of highly concentrated liquid protein solutions. Analytical Biochemistry. 2018;561–562:70–88.
Breitsprecher D, Linke P, Schulze A, Söltl F, Garidel P, Blech M. Getting the Full Picture: Predicting the Aggregation propensity of mAbs Using Chemical and Thermal Denaturation on a Single, Fully Automated Platform, Application Note NT-PR-011, 2016:[7 p.].
Söltl F, Derix J, Blech M, Breitsprecher D. Analysis of formulation-dependent colloidal and conformational stability of monoclonal antibodies, Application Note NT-PR-005, 2015:[7 p.].
Seeliger D, Schulz P, Litzenburger T, Spitz J, Hoerer S, Blech M, et al. Boosting antibody developability through rational sequence optimization. mAbs. 2015;7(3):505–15.
Bergemann K, Eckermann C, Garidel P, Grammatikos S, Jacobi A, Kaufmann H, et al. Production and downstream processing. Handbook of therapeutic antibodies 2007. p. 199–237.
Garidel P, Karow AR, Blech M. Orthogonal spectroscopic techniques for the early developability assessment of therapeutic protein candidates. Spectrosc Eur. 2014;28(4):9–13.
Eftink MR. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys J. 1994;66(2):482–501.
Garidel P, Hegyi M, Bassarab S, Weichel M. A rapid, sensitive and economical assessment of monoclonal antibody conformational stability by intrinsic tryptophan fluorescence spectroscopy. Biotechnol J. 2008;3(9–10):1201–11.
Privalov PL, Potekhin SA. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods in Enzymology. 1986;131:4–51.
Serdyuk IN, Zaccai NR, Zaccai J. Methods in molecular biophysics: structure, dynamics, function. Cambridge University Press; 2007. p. 194–220.
Færgeman NJ, Sigurskjold BW, Kragelund BB, Andersen KV, Knudsen J. Thermodynamics of ligand binding to acyl-coenzyme a binding protein studied by titration Calorimetry. Biochemistry. 1996;35(45):14118–26.
Gummadi SN. What is the role of thermodynamics on protein stability. Biotechnol Bioprocess Eng. 2003;8(9):9–18.
Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech. 2010;21(4):167–93.
Meuzelaar H, Tros M, Huerta-Viga A, van Dijk CN, Vreede J, Woutersen S. Solvent-exposed salt bridges influence the kinetics of α-Helix folding and unfolding. The Journal of Physical Chemistry Letters. 2014;5(5):900–4.
Doig AJ, Sternberg MJE. Side-chain conformational entropy in protein folding. Protein Sci. 1995;4(11):2247–51.
Makhatadze GI, Privalov PL. Energetics of Protein Structure. Advances in Protein Chemistry 1995. p. 307–425.
Southall NT, Dill KA, Haymet A. A view of the hydrophobic effect. ACS Publications; 2002.
Garber E, Demarest SJ. A broad range of fab stabilities within a host of therapeutic IgGs. Biochem Biophys Res Commun. 2007;355(3):751–7.
Lowe D, Dudgeon K, Rouet R, Schofield P, Jermutus L, Christ D. Aggregation, stability, and formulation of human antibody therapeutics. Advances in protein chemistry and structural biology. 84: Elsevier; 2011. p. 41–61.
Shimba N, Torigoe H, Takahashi H, Masuda K, Shimada I, Arata Y, et al. Comparative thermodynamic analyses of the Fv, fab* and fab and fab fragments of anti-dansyl mouse monoclonal antibody. FEBS Lett. 1995;360(3):247–50.
Ionescu RM, Vlasak J, Price C, Kirchmeier M. Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J Pharm Sci. 2008;97(4):1414–26.
Andersen CB, Manno M, Rischel C, Thórólfsson M, Martorana V. Aggregation of a multidomain protein : a coagulation mechanism governs aggregation of a model IgG1 antibody under weak thermal stress. Protein Sci. 2010;19(2):279–90.
Sahin E, Grillo AO, Perkins MD, Roberts CJ. Comparative effects of pH and ionic strength on protein-protein interactions, unfolding, and aggregation for IgG1 antibodies. J Pharm Sci. 2010;99(12):4830–48.
Brummitt RK, Nesta DP, Chang L, Chase SF, Laue TM, Roberts CJ. Nonnative aggregation of an IgG1 antibody in acidic conditions: part 1. Unfolding, colloidal interactions, and formation of high-molecular-weight aggregates. J Pharm Sci. 2011;100(6):2087–103.
Wu H, Kroe-Barrett R, Singh S, Robinson AS, Roberts CJ. Competing aggregation pathways for monoclonal antibodies. FEBS Lett. 2014;588(6):936–41.
Brader ML, Estey T, Bai S, Alston RW, Lucas KK, Lantz S, et al. Examination of thermal unfolding and aggregation profiles of a series of developable therapeutic monoclonal antibodies. Mol Pharm. 2015;12(4):1005–17.
Andrews JM, Roberts CJ. A Lumry-Eyring nucleated polymerization model of protein aggregation kinetics: 1. Aggregation with pre-equilibrated unfolding. J Phys Chem B. 2007;111(27):7897–913.
Lumry R, Eyring H. Conformation changes of proteins. J Phys Chem. 1954;58(2):110–20.
Roberts CJ. Non-native protein aggregation kinetics. Biotechnol Bioeng. 2007;98(5):927–38.
Singla A, Bansal R, Joshi V, Rathore AS. Aggregation kinetics for IgG1-based monoclonal antibody therapeutics. AAPS J. 2016;18(3):689–702.
Seelig J. Cooperative protein unfolding. A statistical-mechanical model for the action of denaturants. Biophys Chem. 2018;233:19–25.
Author information
Authors and Affiliations
Contributions
Richard Melien designed and Michaela Blech designed and supervised the study. Experimental research, data analysis, and evaluation was performed by Richard Melien. All authors contributed to the data analysis. The first draft was written by Richard Melien and was reviewed by Patrick Garidel, Dariush Hinderberger, and Michaela Blech.
Corresponding author
Additional information
Guest Editors: Ahmed Besheer and Hanns-Christian Mahler
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 398 kb)
Rights and permissions
About this article
Cite this article
Melien, R., Garidel, P., Hinderberger, D. et al. Thermodynamic Unfolding and Aggregation Fingerprints of Monoclonal Antibodies Using Thermal Profiling. Pharm Res 37, 78 (2020). https://doi.org/10.1007/s11095-020-02792-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02792-1