ABSTRACT
Purpose
Study the impact of CXCL13 neutralization on germinal center (GC) response in vivo, and build quantitative relationship between target coverage and pharmacological effects at the target tissue.
Methods
An anti-CXCL13 neutralizing monoclonal antibody was dosed in vivo in a T-dependent mouse immunization (TDI) model. A quantitative site-of-action (SoA) model was developed to integrate antibody PK and total CXCL13 levels in serum and spleen towards estimating target coverage as a function of dose. To aid in the SoA model development, a radio-labeled study using [I125] CXCL13 was conducted in mice. Model estimated target coverage was linked to germinal center response using a sigmoidal inhibitory effect model.
Results
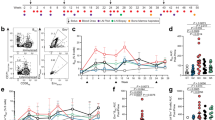
In vivo studies demonstrated that CXCL13 inhibition led to an architectural change in B-cell follicles, dislocation of GCs and a significant reduction in the GC absolute numbers per square area (GC/mm2). The SoA modeling analysis indicated that ~79% coverage in spleen was required to achieve 50% suppression of GC/mm2. The 3 mg/kg dose with 52% spleen coverage resulted in no PD suppression, whereas 30 mg/kg with 93% coverage achieved close to maximum PD suppression, highlighting the steepness of PD response.
Conclusions
This study showcases an application of SoA modeling towards a quantitative understanding of CXCL13 pharmacology.






Similar content being viewed by others
Abbreviations
- CXCL13:
-
CXC chemokine 13
- ELISA:
-
Enzyme-linked immunosorbent assay
- GC:
-
Germinal center
- GC/mm2 :
-
Germinal centers per mm2
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- SoA:
-
Site of action
- TDI:
-
T-dependent immunization model
REFERENCES
Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–14.
Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–93.
Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187(4):655–60.
Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192(11):1553–62.
Amft N, Curnow SJ, Scheel-Toellner D, Devadas A, Oates J, Crocker J, et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren’s syndrome. Arthritis Rheum. 2001;44(11):2633–41.
Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain J Neurol. 2006;129(Pt 1):200–11.
Rosengren S, Wei N, Kalunian KC, Kavanaugh A, Boyle DL. CXCL13: a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis. Rheumatology (Oxford, England). 2011;50(3):603–10.
Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 2008;74(4):448–57.
Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol (Zurich, Switzerland). 2004;14(2):164–74.
Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE, et al. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol (Baltimore, Md : 1950). 2001;166(1):650–5.
Lee HT, Shiao YM, Wu TH, Chen WS, Hsu YH, Tsai SF, et al. Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol. 2010;37(1):45–52.
Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA. Effects of CXCL13 inhibition on lymphoid follicles in models of autoimmune disease. Eur J Clin Investig. 2013;43(5):501–9.
Kamens JS. InventorCXCL13 binding proteins. USA patent US 2008/0227704 A1. 2008.
Chan PL, Jacqmin P, Lavielle M, McFadyen L, Weatherley B. The use of the SAEM algorithm in MONOLIX software for estimation of population pharmacokinetic-pharmacodynamic-viral dynamics parameters of maraviroc in asymptomatic HIV subjects. J Pharmacokinet Pharmacodyn. 2011;38(1):41–61.
Gibiansky L, Gibiansky E. Target-mediated drug disposition model for drugs that bind to more than one target. J Pharmacokinet Pharmacodyn. 2010;37(4):323–46.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35(5):573–91.
Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72(1):1–10.
Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22(10):1589–96.
Bocci V. Interleukins. Clinical pharmacokinetics and practical implications. Clin Pharmacokinet. 1991;21(4):274–84.
Blick M, Sherwin SA, Rosenblum M, Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987;47(11):2986–9.
Gibiansky L, Frey N. Linking interleukin-6 receptor blockade with tocilizumab and its hematological effects using a modeling approach. J Pharmacokinet Pharmacodyn. 2012;39(1):5–16.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72(2):306–20.
Ng CM, Stefanich E, Anand BS, Fielder PJ, Vaickus L. Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm Res. 2006;23(1):95–103.
Betts AM, Clark TH, Yang J, Treadway JL, Li M, Giovanelli MA, et al. The application of target information and preclinical pharmacokinetic/pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp Ther. 2010;333(1):2–13.
Luu KT, Bergqvist S, Chen E, Hu-Lowe D, Kraynov E. A model-based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target-mediated drug disposition. J Pharmacol Exp Ther. 2012;341(3):702–8.
Vugmeyster Y, Rohde C, Perreault M, Gimeno RE, Singh P. Agonistic TAM-163 antibody targeting Tyrosine kinase receptor-B: applying mechanistic modeling to enable preclinical to clinical translation and guide clinical trial design. mAbs. 2013;5(3):373–83.
Wang B, Lau YY, Liang M, Vainshtein I, Zusmanovich M, Lu H, et al. Mechanistic modeling of antigen sink effect for mavrilimumab following intravenous administration in patients with rheumatoid arthritis. J Clin Pharmacol. 2012;52(8):1150–61.
Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206(5):1029–36.
Hsei V, Deguzman GG, Nixon A, Gaudreault J. Complexation of VEGF with bevacizumab decreases VEGF clearance in rats. Pharm Res. 2002;19(11):1753–6.
Vugmeyster Y, DeFranco D, Szklut P, Wang Q, Xu X. Biodistribution of [125I]-labeled therapeutic proteins: application in protein drug development beyond oncology. J Pharm Sci. 2010;99(2):1028–45.
Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J. 2011;13(1):99–110.
Staack RF, Jordan G, Heinrich J. Mathematical simulations for bioanalytical assay development: the (un-)necessity and (im-)possibility of free drug quantification. Bioanalysis. 2012;4(4):381–95.
ACKNOWLEDGMENTS AND DISCLOSURES
Authors wish to thank Quintus Medley, Ph.D. and Jill Wright, Ph.D. for reviewing the manuscript and proving useful suggestions.
This study was supported by Pfizer, Inc. All authors were employees of Pfizer at the time of study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 46 kb)
Rights and permissions
About this article
Cite this article
Brodfuehrer, J., Rankin, A., Edmonds, J. et al. Quantitative Analysis of Target Coverage and Germinal Center Response by a CXCL13 Neutralizing Antibody in a T-Dependent Mouse Immunization Model. Pharm Res 31, 635–648 (2014). https://doi.org/10.1007/s11095-013-1185-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1185-2