ABSTRACT
Purpose
To formulate nanoemulsions (NE) with potential for delivering poorly water-soluble drugs to the lungs.
Method
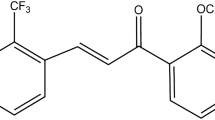
A self nanoemulsifying composition consisting of cremophor RH 40, PEG 400 and labrafil M 2125 CS was selected after screening potential excipients. The solubility of carbamazepine, a poorly water-soluble drug, was tested in the formulation components. Oil-in-water (o/w) NEs were characterized using dynamic light scattering, electrophoretic light scattering, transmission electron microscopy (TEM) and differential scanning calorimetry. NEs were nebulized into a mist using a commercial nebulizer and characterized using laser diffraction and TEM. An aseptic method was developed for preparing sterile NEs. Biocompatibility of the formulation was evaluated on NIH3T3 cells using MTT assay. In vitro permeability of the formulation was tested in zebra fish eggs, HeLa cells, and porcine lung tissue.
Results
NEs had neutrally charged droplets of less than 20 nm size. Nebulized NEs demonstrated an o/w nanostructure. The mist droplets were of size less than 5 μm. Sterility testing and cytotoxicity results validated that the NE was biocompatible and sterile. In vitro tests indicated oil nanodroplets penetrating intracellularly through biological membranes.
Conclusion
The nanoemulsion mist has the potential for use as a pulmonary delivery system for poorly water-soluble drugs.











Similar content being viewed by others
Abbreviations
- CBZ:
-
carbamazepine
- CLSM:
-
confocal laser scanning microscope
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DLS:
-
dynamic light scattering
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- ELS:
-
electrophoretic light scattering
- FBS:
-
fetal bovine serum
- FTM:
-
fluid thioglycollate medium
- He-Ne laser:
-
Helium-Neon laser
- km ratio:
-
surfactant/co-surfactant ratio
- MH:
-
Mueller Hinton
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NE:
-
nanoemulsions
- OCT:
-
optimal cutting temperature
- PBS:
-
phosphate buffered saline
- SCD:
-
soybean casein digest medium
- SNE:
-
self-nanoemulsifying
- SNEDDS:
-
self-nanoemulsifying drug delivery system
References
Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm. 2002;235(1–2):247–65.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227–43.
Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci. 2009;330(2):443–8.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Kawakami K, Yoshikawa T, Hayashi T, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: II. In vivo study. J Control Release. 2002;81(1–2):75–82.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26.
Stevens RE, Limsakun T, Evans G, Mason Jr DH. Controlled, multidose, pharmacokinetic evaluation of two extended-release carbamazepine formulations (Carbatrol and Tegretol-XR). J Pharm Sci. 1998;87(12):1531–4.
El-Zein H, Riad L, El-Bary AA. Enhancement of carbamazepine dissolution: in vitro and in vivo evaluation. Int J Pharm. 1998;168(2):209–20.
Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60(6):717–33.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74.
Hoover JL, Rush BD, Wilkinson KF, Day JS, Burton PS, Vidmar TJ, et al. Peptides are better absorbed from the lung than the gut in the rat. Pharm Res. 1992;9(8):1103–6.
Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH, Khang G, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274(1–2):65–73.
Craig DQM, Lievens HSR, Pitt KG, Storey DE. An investigation into the physico-chemical properties of self-emulsifying systems using low frequency dielectric spectroscopy, surface tension measurements and particle size analysis. Int J Pharm. 1993;96(1–3):147–55.
United States Pharmacopeial Convention. The United States pharmacopeia: the national formulary. Rockville: United States Pharmacopeial Convention; 2006.
Nesamony J, Zachar CL, Jung R, Williams FE, Nauli S. Preparation, characterization, sterility validation, and in vitro cell toxicity studies of microemulsions possessing potential parenteral applications. Drug Dev Ind Pharm. 2012;5:5.
Kumar S, Singh HN. Competitive solubilization of Sudan IV and anthracene in micellar systems. Colloids Surf. 1992;69(1):1–4.
Krishna SM, Seto SW, Moxon JV, Rush C, Walker PJ, Norman PE, et al. Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am J Pathol. 2012;181(2):706–18.
Rebane R, Leito I, Yurchenko S, Herodes K. A review of analytical techniques for determination of Sudan I-IV dyes in food matrixes. J Chromatogr A. 2010;1217(17):2747–57.
Behrens I, Pena AIV, Alonso MJ, Kissel T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: the effect of mucus on particle adsorption and transport. Pharm Res. 2002;19(8):1185–93.
Hahnenkamp I, Graubner G, Gmehling J. Measurement and prediction of solubilities of active pharmaceutical ingredients. Int J Pharm. 2010;388(1–2):73–81.
Abid SK, Hamid SM, Sherrington DC. Micellization and surface-activity of long-chain monoquaternary and diquaternary ammonium salts. J Colloid Interface Sci. 1987;120(1):245–55.
Kibbe AH. Handbook of pharmaceutical excipients. Washington, DC: American Pharmaceutical Association; 2000.
Li G, Fan Y, Li X, Wang X, Li Y, Liu Y, et al. In vitro and in vivo evaluation of a simple microemulsion formulation for propofol. Int J Pharm. 2012;425(1–2):53–61.
Shinoda K, Shibata Y, Lindman B. Interfacial tensions for lecithin microemulsions including the effect of surfactant and polymer addition. Langmuir. 1993;9(5):1254–7.
Kahlweit M, Strey R, Schomaecker R, Haase D. General patterns of the phase behavior of mixtures of water, nonpolar solvents, amphiphiles, and electrolytes. 2. Langmuir. 1989;5(2):305–15.
Sha X, Yan G, Wu Y, Li J, Fang X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur J Pharm Sci. 2005;24(5):477–86.
Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, et al. Microemulsion formulation for enhanced absorption of poorly soluble drugs I. Prescription design. J Control Release. 2002;81(1–2):65–74.
Gershanik T, Benzeno S, Benita S. Interaction of a self-emulsifying lipid drug delivery system with the everted rat intestinal mucosa as a function of droplet size and surface charge. Pharm Res. 1998;15(6):863–9.
Adjei A, Garren J. Pulmonary delivery of peptide drugs: effect of particle size on bioavailability of leuprolide acetate in healthy male volunteers. Pharm Res. 1990;7(6):565–9.
Kotyla T, Kuo F, Moolchandani V, Wilson T, Nicolosi R. Increased bioavailability of a transdermal application of a nano-sized emulsion preparation. Int J Pharm. 2008;347(1–2):144–8.
Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212(2):233–46.
Gabizon A, Papahadjopoulos D. The role of surface charge and hydrophilic groups on liposome clearance in vivo. Biochim Biophys Acta. 1992;10(1):94–100.
Park K-M, Kim C-K. Preparation and evaluation of flurbiprofen-loaded microemulsion for parenteral delivery. Int J Pharm. 1999;181(2):173–9.
Kotmakchiev M, Kantarci G, Cetintas VB, Ertan G. Cytotoxicity of a novel oil/water microemulsion system loaded with mitomycin-C in in vitro lung cancer models. Drug Dev Res. 2012;73(4):185–95.
Katzhendler I, Azoury R, Friedman M. The effect of egg albumin on the crystalline properties of carbamazepine in sustained release hydrophilic matrix tablets and in aqueous solutions. J Control Release. 2000;65(3):331–43.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23–24):1068–75.
Zhang D, Hamilton PD, Kao JLF, Venkataraman S, Wooley KL, Ravi N. Formation of nanogel aggregates by an amphiphilic cholesteryl-poly(amidoamine) dendrimer in aqueous media. J Polymer Sci, Part A: Polymer Chem. 2007;45(12):2569–75.
Sintov AC, Levy HV, Botner S. Systemic delivery of insulin via the nasal route using a new microemulsion system: In vitro and in vivo studies. J Control Release. 2010;148(2):168–76.
Elversson J, Millqvist-Fureby A, Alderborn G, Elofsson U. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J Pharm Sci. 2003;92(4):900–10.
Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868–71.
Nesamony J, Zachar CL, Jung R, Williams FE, Nauli S. Preparation, characterization, sterility validation, and in vitro cell toxicity studies of microemulsions possessing potential parenteral applications. Drug Dev Ind Pharm. 2013;39(2):240–51.
Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56(3):279–85.
Lo J-T, Chen B-H, Lee T-M, Han J, Li J-L. Self-emulsifying O/W formulations of paclitaxel prepared from mixed nonionic surfactants. J Pharm Sci. 2010;99(5):2320–32.
Bonsignorio D, Perego L, Del Giacco L, Cotelli F. Structure and macromolecular composition of the zebrafish egg chorion. Zygote. 1996;4(2):101–8.
Levitt JM, Baldwin A, Papadakis A, Puri S, Xylas J, Munger K, et al. Intrinsic fluorescence and redox changes associated with apoptosis of primary human epithelial cells. J Biomed Opt. 2006;11(6):064012/1–/10.
Rothkotter H-J. Anatomical particularities of the porcine immune system–a physician’s view. Dev Comp Immunol. 2009;33(3):267–72.
Williamson DH, Fennell DJ. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–51.
Acknowledgments and Disclosures
This research was performed with support from start-up funds made available by the Department of Pharmacy Practice at the University of Toledo College of Pharmacy and Pharmaceutical Sciences. We are grateful to Dr. Joseph Lawrence, Center for Sensor and Materials Characterization, University of Toledo College of Engineering for his assistance during the TEM work. We also thank Ms. Maki Takahashi, Department of Pharmacology, University of Toledo College of Pharmacy and Pharmaceutical Sciences for her help with cell cultures.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental figure 1
Schematic diagram depicting generation of the nanostructured mist (JPEG 9 kb)
Supplemental figure 2
3-day CLSM images obtained by virtual optical cross sectioning through a zebra fish egg exposed to 100 mg/ml Sudan IV loaded NE for 6 h (JPEG 11 kb)
Rights and permissions
About this article
Cite this article
Nesamony, J., Kalra, A., Majrad, M.S. et al. Development and Characterization of Nanostructured Mists with Potential for Actively Targeting Poorly Water-Soluble Compounds into the Lungs. Pharm Res 30, 2625–2639 (2013). https://doi.org/10.1007/s11095-013-1088-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1088-2