Abstract
Purpose
Approximately 50% of active pharmaceutical ingredients (APIs) are manufactured and formulated as salts, due to their enhanced dissolution rates or improved solid state properties. It is essential to maintain the appropriate solid state form of the drug during processing and over the lifetime of the product. The aim of this study was to investigate the contributing factors in the process of disproportionation, whereby the salt converts back to the free form of the drug.
Methods
Infrared and Raman spectroscopy were used to detect and quantify the formation of free base in physical mixtures with excipients. The pH-solubility relationships were determined based on measured salt solubilities and properties of the free form.
Results
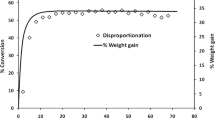
The mesylate salts of two model pharmaceutical compounds were found to disproportionate to the free base form when physically mixed with certain common basic excipients and exposed to moderate relative humidities. In contrast, the napsylate salts were much more resistant to disproportionation. The napsylate salts had solubilities more than 3 orders of magnitude lower than the respective mesylate salts, and showed little to no detectable formation of free base. The mesylate salts with higher solubilities showed significant levels of conversion to the free base.
Conclusions
It appears that both the solubility and pHmax (the pH of a solution where there is saturation of both ionized and unionized species) of the salts, as well as the base solubility, play important roles in determining the susceptibility of salts to disproportionate. The extent of conversion was also affected by excipient properties, including basicity, solubility, physical state and surface area.










Similar content being viewed by others
References
Rohrs BR, Thamann TJ, Gao P, Stelzer DJ, Bergren MS, Chao RS. Tablet dissolution affected by a moisture mediated solid-state interaction between drug and disintegrant. Pharm. Res. 1999;16:1850–56.
Zannou EA, Ji Q, Joshi YM, Serajuddin ATM. Stabilization of the maleate salt of a basic drug by adjustment of microenvironmental pH in solid dosage form. Int. J. Pharm. 2007;337:210–8.
Williams AC, Cooper VB, Thomas L, Griffith LJ, Petts CR, Booth SW. Evaluation of drug physical form during granulation, tabletting and storage. Int. J. Pharm. 2004;275:29–39.
Towler CS, Li TL, Wikstrom H, Remick DM, Sanchez-Felix MV, Taylor LS. An Investigation into the Influence of Counterion on the Properties of Some Amorphous Organic Salts. Mol. Pharm. 2008;5:946–55.
Guo YS, Byrn SR, Zografi G. Effects of lyophilization on the physical characteristics and chemical stability of amorphous quinapril hydrochloride. Pharm. Res. 2000;17:930–5.
Adeyeye MC and Brittain HG (eds.). Preformulation in Solid Dosage Form Development, Informa Healthcare, 2008.
Salameh AK, Taylor LS. Deliquescence in binary mixtures. Pharm. Res. 2005;22:318–24.
Box KJ, Comer JEA. Using Measured pK(a) LogP and Solubility to Investigate Supersaturation and Predict BCS Class. Curr. Drug Metab. 2008;9:869–78.
Piel G, Evrard B, Fillet M, Llabres G, Delattre L. Development of a non-surfactant parenteral formulation of miconazole by the use of cyclodextrins. Int. J. Pharm. 1998;169:15–22.
Black SN, Collier EA, Davey RJ, Roberts RJ. Structure, solubility, screening, and synthesis of molecular salts. J. Pharm. Sci. 2007;96:1053–68.
Avila CM, Martinez F. Thermodynamic study of the solubility of benzocaine in some organic and aqueous solvents. J. Solution Chem. 2002;31:975–85.
Zografi G, Hancock B. Water-Solid Interactions in Pharmaceutical Systems. In: Crommelin DJA, Midha KK, Nagai T, editors. Topics in Pharmaceutical Sciences. Stuttgart, Germany: Proceedings of International Congress on Pharmaceutical Sciences FIP; 1993. p. 405–19.
Sharpe SA, Celik M, Newman AW, Brittain HG. Physical characterization of the polymorphic variations of magnesium stearate and magnesium palmitate hydrate species. Struct. Chem. 1997;8(1):73–84.
Skoog DA, Holler FJ, Nieman TA. Principles of Instrumental Analysis. 5th ed. Philadelphia: Saunders College Publishing; 1998.
Ross KD. Estimation of Water Activity in Intermediate Moisture Foods. Food Technol. 1975;29:26–34.
Dai DJ, Peters SJ, Ewing GE. Water-Adsorption and Dissociation on Nacl Surfaces. J. Phys. Chem. 1995;99:10299–304.
Luna M, Rieutord F, Melman NA, Dai Q, Salmeron M. Adsorption of water on alkali halide surfaces studied by scanning polarization force microscopy. J. Phys. Chem. A. 1998;102:6793–800.
Shindo H, Ohashi M, Baba K, Seo A. AFM observation of monatomic step movements on NaCl(001) with the help of adsorbed water. Surf. Sci. 1996;358:111–4.
Shindo H, Ohashi M, Tateishi O, Seo A. Atomic force microscopic observation of step movements on NaCl(001) and NaF(001) with the help of adsorbed water. J. Chem. Soc., Faraday Trans. 1997;93:1169–74.
Verdaguer A, Sacha GM, Luna M, Ogletree DF and Salmeron M. Initial stages of water adsorption on NaCl (100) studied by scanning polarization force microscopy. J. Chem. Phy. 123: (2005).
Kramer SF and Flynn GL. Solubility of Organic Hydrochlorides. J. Pharm. Sci.. 61:1896-& (1972).
Bogardus JB, Blackwood RK. Solubility of Doxycycline in Aqueous-Solution. J. Pharm. Sci. 1979;68:188–94.
Serajuddin ATM. Salt formation to improve drug solubility. Adv. Drug Delivery Rev. 2007;59:603–16.
Stahl PH, Wemuth CG. Handbook of Pharmaceutical Salts. New York: Wiley-VCH; 2002.
Govindarajan R, Zinchuk A, Hancock B, Shalaev E, Suryanarayanan R. Ionization states in the microenvironment of solid dosage forms: Effect of formulation variables and processing. Pharm. Res. 2006;23:2454–68.
Pudipeddi M, Zannou EA, Vasanthavada M, Dontabhaktuni A, Royce AE, Josh YM, et al. Measurement of surface pH of pharmaceutical solids: A critical evaluation of indicator dye-sorption method and its comparison with slurry pH method. J. Pharm. Sci. 2008;97:1831–42.
Dulin WA. Degradation of bisoprolol fumarate in tablets formulated with dicalcium phosphate. Drug Dev. Ind. Pharm. 1995;21:393–409.
Glombitza BW, Oelkrug D, Schmidt PC. Surface-acidity of solid pharmaceutical excipients 1. Determination of the surface-acidity. Eur. J. Pharm. Biopharm. 1994;40:289–93.
Glombitza BW, Schmidt PC. Surface-acidity of solid pharmaceutical excipients 2. Effect of the surface-acidity on the decomposition rate of acetylsalicylic acid. Eur. J. Pharm. Biopharm. 1995;41:114–9.
Hussain MSH, York P, Timmins P. A study of the formation of magnesium stearate film on sodium-chloride using energy-dispersive x-ray-analysis. Int. J. Pharm. 1988;42:89–95.
Acknowledgements
The authors acknowledge AstraZeneca R&D Lund Sweden for funding this research. Dr. Kjell Jarring is sincerely thanked for helpful discussions. Aansh jarmarwala is thanked for his help in collecting spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerrieri, P., Taylor, L.S. Role of Salt and Excipient Properties on Disproportionation in the Solid-State. Pharm Res 26, 2015–2026 (2009). https://doi.org/10.1007/s11095-009-9918-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9918-y