Abstract
Purpose
Potentiometric lipid membrane–water partition coefficient studies neglect electrostatic interactions to date; this leads to incorrect results. We herein show how to account properly for such interactions in potentiometric data analysis.
Materials and Methods
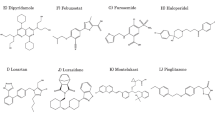
We conducted potentiometric titration experiments to determine lipid membrane–water partition coefficients of four illustrative drugs, bupivacaine, diclofenac, ketoprofen and terbinafine. We then analyzed the results conventionally and with an improved analytical approach that considers Coulombic electrostatic interactions.
Results
The new analytical approach delivers robust partition coefficient values. In contrast, the conventional data analysis yields apparent partition coefficients of the ionized drug forms that depend on experimental conditions (mainly the lipid-drug ratio and the bulk ionic strength). This is due to changing electrostatic effects originating either from bound drug and/or lipid charges. A membrane comprising 10 mol-% mono-charged molecules in a 150 mM (monovalent) electrolyte solution yields results that differ by a factor of 4 from uncharged membranes results.
Conclusion
Allowance for the Coulombic electrostatic interactions is a prerequisite for accurate and reliable determination of lipid membrane–water partition coefficients of ionizable drugs from potentiometric titration data. The same conclusion applies to all analytical methods involving drug binding to a surface.






Similar content being viewed by others
Notes
When P N is close to P I, the wrong assumption of a constant P I can also affect the calculated P N value, as the two parameters are typically derived simultaneously from the same data set.
Data from another study (not published yet) conducted in our laboratory.
This is also evident for terbinafine, although the conventional model, neglecting electrostatic interactions, could not be used to obtain P I values. The \(pK^{{app}}_{a} \)values at the same lipid concentrations were higher at higher bulk ionic strength, which indicates higher apparent P I (data not shown).
References
C. J. Alcorn, R. J. Simpson, D. E. Leahy, and T. J. Peters. Partition and distribution coefficients of solutes and drugs in brush border membrane vesicles. Biochem. Pharmacol. 45:1775–1782 (1993). doi:10.1016/0006-2952(93)90433-W.
R. P. Austin, A. M. Davis, and C. N. Manners. Partitioning of ionizing molecules between aqueous buffers and phospholipid vesicles. J. Pharm. Sci. 84:1180–1183 (1995). doi:10.1002/jps.2600841008.
A. Avdeef. Absorption and drug development: solubility, permeability, and charge state. Wiley, New Jersey, 2003.
R. P. Austin, P. Barton, A. M. Davis, C. N. Manners, and M. C. Stansfield. The effect of ionic strength on liposome-buffer and 1-octanol-buffer distribution coefficients. J. Pharm. Sci. 87:599–607 (1998). doi:10.1021/js9703481.
A. Avdeef, K. J. Box, J. E. Comer, C. Hibbert, and K. Y. Tam. pH-metric logP 10. Determination of liposomal membrane–water partition coefficients of ionizable drugs. Pharm. Res. 15:209–215 (1998). doi:10.1023/A:1011954332221.
B. I. Escher, R. Schwarzenbach, and J. C. Westall. Evaluation of liposome–water partitioning of organic acids and bases. 2. Comparison of experimental determination methods. Environ. Sci. Technol. 34:3962–3968 (2000). doi:10.1021/es0010711.
S. D. Krämer, J. C. Gautier, and P. Saudemon. Considerations on the potentiometric log P determination. Pharm. Res. 15:1310–1313 (1998). doi:10.1023/A:1011968630713.
C. Ottiger, and H. Wunderli-Allenspach. Partition behaviour of acids and bases in a phosphatidylcholine liposome–buffer equilibrium dialysis system. Eur. J. Pharm. Sci. 5:223–231 (1997). doi:10.1016/S0928–0987(97)00278–9.
A. V. Thomae, T. Koch, C. Panse, H. Wunderli-Allenspach, and S. D. Kramer. Comparing the lipid membrane affinity and permeation of drug-like acids: The intriguing effects of cholesterol and charged lipids. Pharm. Res. 24:1457–1472 (2007). doi:10.1007/s11095-007-9263-y.
B. de Castro, P. Gameiro, J. L. F. C. Lima, C. Matos, and S. Reis. A fast and reliable spectroscopic method for the determination of membrane–water partition coefficients of organic compounds. Lipids. 36:89–96 (2001). doi:10.1007/s11745-01-673-0.
C. Rodrigues, P. Gameiro, S. Reis, J. L. F. C. Lima, and B. de castro. Derivative spectrophotometry as a tool for the determination of drug partition coefficients in water/dimyristoyl-α-phosphatidylglycerol (DMPG) liposomes. Biophys. Chem. 94:97–106 (2001). doi:10.1016/S0301-4622(01)00227-7.
N. C. Santos, M. Prieto, and M. A. R. B. Castanho. Quantifying molecular partition into model systems of biomembranes: an emphasis on optical spectroscopic methods. Biochim. Biophys. Acta. 1612:123–135 (2003). doi:10.1016/S0005-2736(03)00112-3.
R. P. Austin, P. Barton, A. M. Davis, R. E. Fessey, and M. C. Wenlock. The thermodynamics of the partitioning of ionizing molecules between aqueous buffers and phospholipid membranes. Pharm. Res. 22:1649–1657 (2005). doi:10.1007/s11095-005-6336-7.
X. Y. Liu, Q. Yang, N. Kamo, and J. Miyake. Effect of liposome type and membrane fluidity on drug–membrane partitioning analyzed by immobilized liposome chromatography. J. Chromatogr. A. 913:123–131 (2001). doi:10.1016/S0021-9673(00)01266-8.
Q. Yang, X. Y. Liu, S. Ajiki, M. Hara, P. Lundahl, and J. Miyake. Avidin–biotin immobilization of unilamellar liposomes in gel beads for chromatographic analysis of drug–membrane partitioning. J. Chromatogr. B Biomed. Sci. Appl. 707:131–141 (1998). doi:10.1016/S0378-4347(97)00620-8.
C. Matos, B. de Castro, P. Gameiro, J. L. Lima, and S. Reis. Zeta-potential measurements as a tool to quantify the effect of charged drugs on the surface potential of egg phosphatidylcholine liposomes. Langmuir. 20:369–377 (2004). doi:10.1021/la034780b.
M. Ikonen, L. Murtomäki, and K. Kontturia. An electrochemical method for the determination of liposome–water partition coefficients of drugs. J. Electroanal. Chem. 602:189–194 (2007). doi:10.1016/j.jelechem.2006.12.014.
T. Hata, T. Sakamoto, H. Matsuki, and S. Kaneshina. Partition coefficients of charged and uncharged local anesthetics into dipalmitoylphosphatidylcholine bilayer membrane: estimation from pH dependence on the depression of phase transition temperatures. Colloids Surf. B Biointerfaces. 22:77–84 (2001). doi:10.1016/S0927-7765(01)00160-6.
A. Hildebrand, K. Beyer, R. Neubert, P. Garidel, and A. Blume. Temperature dependence of the interaction of cholate and deoxycholate with fluid model membranes and their solubilization into mixed micelles. Colloids Surf. B: Biointerfaces. 32:335–351 (2003). doi:10.1016/j.colsurfb.2003.08.001.
K. Balon, B. U. Riebesehl, and B. W. Muller. Drug liposome partitioning as a tool for the prediction of human passive intestinal absorption. Pharm. Res. 16:882–888 (1999). doi:10.1023/A:1018882221008.
J. Lasch. Interaction of detergents with lipid vesicles. Biochim. Biophys. Acta. 1241:269–292 (1995).
F. H. Clarke. Ionization constants by curve fitting: application to the determination of partition coefficients. J. Pharm. Sci. 73:226–230 (1984). doi:10.1002/jps.2600730221.
F. H. Clarke, and N. M. Cahoon. Ionization constants by curve fitting: determination of partition and distribution coefficients of acids and bases and their ions. J. Pharm. Sci. 76:611–620 (1987). doi:10.1002/jps.2600760806.
S. Ohki. Adsorption of local anesthetics on phospholipid membranes. Biochim. Biophys. Acta. 777:56–66 (1984). doi:10.1016/0005-2736(84)90496-6.
P. G. Thomas, and J. Seelig. Binding of the calcium antagonist flunarizine to phosphatidylcholine bilayers: charge effects and thermodynamics. Biochem. J. 291:397–402 (1993).
M. S. Fernandez, and P. Fromherz. Lipoid pH indicators as probes of electrical potential and polarity in micells. J. Phys. Chem. 81:1755–1761 (1977). doi:10.1021/j100533a009.
E. Kuchinka, and J. Seelig. Interaction of melittin with phosphatidylcholine membranes. Binding isotherm and lipid head-group conformation. Biochemistry. 28:4216–4221 (1989). doi:10.1021/bi00436a014.
H. D. Bäuerle, and J. Seelig. Interaction of charged and uncharged calcium channel antagonists with phospholipid membranes. Binding equilibrium, binding enthalpy, and membrane location. Biochemistry. 30:7203–7211 (1991). doi:10.1021/bi00243a023.
A. Hildebrand, R. Neubert, P. Garidel, and A. Blume. Bile salt induced solubilization of synthetic phosphatidylcholine vesicles studied by isothermal titration calorimetry. Langmuir. 18:2836–2847 (2002). doi:10.1021/la011421c.
A. Seelig, P. R. Allegrini, and J. Seelig. Partitioning of local anesthetics into membranes: surface charge effects monitored by the phospholipid head-group. Biochim. Biophys. Acta. 939:267–276 (1988). doi:10.1016/0005-2736(88)90070-3.
P. Friberger, and G. Aberg. Some physiochemical properties of the racemates and the optically active isomers of two local anaesthetic compounds. Acta Pharm. Suec. 8:361–364 (1971).
M. Meloun, S. Bordovská, and L. Galla. The thermodynamic dissociation constants of four non-steroidal anti-inflammatory drugs by the least-squares nonlinear regression of multiwavelength spectrophotometric pH-titration data. J. Pharm. Biomed. Anal. 45:552–564 (2007). doi:10.1016/j.jpba.2007.07.029.
A. Avdeef, C. M. Berger, and C. Brownell. pH-metric solubility. 2: correlation between the acid–base titration and the saturation shake-flask solubility–pH methods. Pharm. Res. 17:85–89 (2000). doi:10.1023/A:1007526826979.
J. M. Carrozzino. Drug partitioning and solvation environments in lipid bilayers. Department of Chemistry, North Carolina State University, Raleigh, 2004, p. 224.
K. Ohsawa, and H. Ohshima. Electrophoretic mobility and isoelectric point of purified brush border membrane vesicles. Electrophoresis. 5:148–154 (1984). doi:10.1002/elps.1150050305.
M. Sugawara, H. Oikawa, M. Kobayashi, K. Iseki, and K. Miyazaki. Effect of membrane surface potential on the uptake and the inhibition of cationic compounds in rat intestinal brush-border membrane vesicles and liposomes. Biochim. Biophys. Acta. 1234:22–28 (1995). doi:10.1016/0005-2736(94)00250-S.
C. Engvall, and P. Lundahl. Drug partition chromatography on immobilized porcine intestinal brush border membranes. J. Chromatogr. A. 1031:107–112 (2004). doi:10.1016/j.chroma.2003.11.063.
Cevc. Electrostatic characterization of liposomes. Chem. Phys. Lipids. 64:163–186 (1993). doi:10.1016/0009-3084(93)90064-A.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The intrinsic (lipid-dependent) membrane surface charge density, σ mem, is calculated from membrane composition:
where z L is the charged lipid valence, e 0 the elementary electric charge, x C the molar fraction of the charged lipids with molecular area A C, and A N the average surface area of the neutral lipid molecules. In the current study, we used A N ≈ A C ≈ 0.65 nm2, which is a good approximation for typical fluid-phase phospholipids. During potentiometric titration, the relative proportion of the charged lipids may vary, if the employed pH range overlaps with the lipid titration range. If so, the resulting surface charge density variation must be considered.
The average drug-dependent membrane surface charge density, σ D, is calculated analogously:
z D is the bound drug valence, C L the membrane–forming lipid concentration, \(C^{{\text{I}}}_{{{\text{mem}}}}\) and \(C^{{\text{N}}}_{{{\text{mem}}}}\) the concentrations of the membrane associated ionized and neutral drug forms, respectively. The contribution of the membrane associated drug molecules to the lipid bilayer surface area, A D, is normally relatively small. It can thus be neglected. We can now calculate σ D once \(C^{{\text{I}}}_{{{\text{mem}}}}\) is known.
To calculate \(C^{{\text{I}}}_{{{\text{mem}}}}\), we will start with the total drug concentration C tot = C mem + C aq. Substitution of C aq from Eq. 2 and rearrangement allow calculation of the total membrane bound drug concentration:
The ionized drug fraction, α, is:
Combining Eqs. 6, 11, and 12 yields:
which takes into account the Coulombic electrostatic contributions from both σ D and σ mem. The superscript I denotes the deprotonated form, X, for an acidic drug and the protonated form, XH, for a basic drug (cf. Eqs. 7 and 8). All concentrations are defined relative to the total suspension volume. Combining Eqs. 10 and 14 finally yields the drug-dependent surface charge density:
The procedure is applicable at any pH value and ideally should involve the entire titration curve. σ D is a function of Φ (or ψ) and vice versa, i.e. they are interdependent. The equation must thus be solved in a self-consistent fashion, and typically numerically. (We used Mathcad employing Secant and Mueller method for numerical solving.)
The Debye ion screening length, λ D, is a property of the electrolyte solution and is given for 1:1 electrolytes by:
ε 0 is the permittivity of free space (8.8542 × 10−12 As/Vm), ε r the dielectric constant at the drug binding site (an average value of 40 for the lipid head group was used), k B the Boltzmann constant (1.38 × 10−23 JK−1), T the absolute temperature, e 0 the elementary electric charge (1.602 × 10−19 C), N A Avogadro’s number (6.02205 × 1023 mol−1), C el the bulk molar electrolyte concentration.
The electrostatic potential, ψ, of a uniformly charged surface in contact with a 1:1 electrolyte is given within the framework of Gouy–Chapman approximation (38), as a function of the total surface charge density, σ = σ D + σ mem, by:
σ is the surface charge density in Cm−2 and asinh the inverse hyperbolic sine (areasinushyperbolicus). The normalized dimensionless electrostatic potential, Φ, is defined as the ratio of electrostatic potential energy, ze 0 ψ, and thermal energy, k B T:
Numeric approximations to Eqs. 16 and 18 are given in Table V.
According to the Gouy–Chapman model, the relationship between the electrostatic potential ψ(x) at distance x from a uniformly charged surface and the electrostatic surface potential ψ(x = 0), is:
This provides means for estimating the effective distance between the lipid and the drug charges in a membrane.
The electrostatic correction described in this article allows only for the Coulombic, i.e. charge–charge interactions. Other contributions, such as hydration (polarity) effects, can be influential as well. If they are not small, such interactions should be considered, following the basic, self-consistent approach described in this work.
Rights and permissions
About this article
Cite this article
Elsayed, M.M.A., Vierl, U. & Cevc, G. Accurate Potentiometric Determination of Lipid Membrane–Water Partition Coefficients and Apparent Dissociation Constants of Ionizable Drugs: Electrostatic Corrections. Pharm Res 26, 1332–1343 (2009). https://doi.org/10.1007/s11095-009-9842-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9842-1