Abstract
Purpose
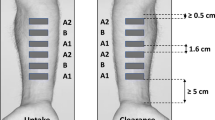
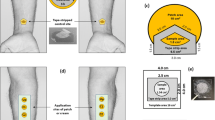
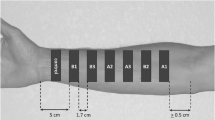
The dermatopharmacokinetic methodology, in which tape stripping of the stratum corneum (SC) is used to access the amount of drug accumulated in the skin barrier, has been proposed for the quantification of topical drug bioavailability. This investigation examined the clearance phase of a model drug from the SC after a short application of an infinite dose.
Methods
A saturated solution of ibuprofen in propylene glycol/water was applied to the forearm of human volunteers for 30 min. The formulation was then removed and the drug profile across the SC was assessed immediately, and over the next 4 h.
Results
The clearance phase depends only on drug diffusivity in the SC. However, the expected, progressive “flattening” of the concentration profiles with increasing time post-formulation removal was not observed. It was subsequently deduced, using infrared spectroscopy, that the rapid percutaneous diffusion of propylene glycol, relative to ibuprofen, resulted in the transient maintenance of a saturated drug concentration at the SC surface even after removal of the original formulation.
Conclusions
The important role of formulation excipients in topical delivery is demonstrated, and the local disposition of cosolvents within the SC may impact significantly on drug dermatopharmacokinetics and local bioavailability.







Similar content being viewed by others
References
HHS, FDA, and CDER. Topical dermatological drug product NDAs and ANDAs—in vivo bioavailability, bioequivalence, in vitro release and associated studies Draft Guidance for Industry. (1998).
Y. N. Kalia, I. Alberti, A. Naik, and R. H. Guy. Assessment of topical bioavailability in vivo: the importance of stratum corneum thickness. Skin Pharmacol Appl Skin Physiol. 14(Suppl 1):82–86 (2001) doi:10.1159/000056394.
L. K. Pershing, J. L. Nelson, J. L. Corlett, S. P. Shrivastava, D. B. Hare, and V. P. Shah. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 48:740–751 (2003) doi:10.1067/mjd.2003.175.
M. B. Reddy, A. L. Stinchcomb, R. H. Guy, and A. L. Bunge. Determining dermal absorption parameters in vivo from tape strip data. Pharm Res. 19:292–298 (2002) doi:10.1023/A:1014443001802.
V. P. Shah. IV-IVC for topically applied preparations—a critical evaluation. Eur J Pharm Biopharm. 60:09–14 (2005) doi:10.1016/j.ejpb.2004.09.015.
E. Benfeldt, S. H. Hansen, A. Volund, T. Menne, and V. P. Shah. Bioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J Invest Dermatol. 127:170–178 (2007) doi:10.1038/sj.jid.5700495.
M. Breternitz, M. Flach, J. Prassler, P. Elsner, and J. W. Fluhr. Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping. A randomized, controlled study. Br J Dermatol. 156:231–240 (2007) doi:10.1111/j.1365-2133.2006.07632.x.
C. Herkenne, A. Naik, Y. N. Kalia, J. Hadgraft, and R. H. Guy. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm Res. 23:1850–1856 (2006) doi:10.1007/s11095-006-9011-8.
C. Herkenne, A. Naik, Y. N. Kalia, J. Hadgraft, and R. H. Guy. Effect of propylene glycol on ibuprofen absorption into human skin in vivo. J Pharm Sci. 97(1):185–197 (2007).
C. Herkenne, A. Naik, Y. N. Kalia, J. Hadgraft, and R. H. Guy. Dermatopharmacokinetic prediction of topical drug bioavailability in vivo. J Invest Dermatol. 127:887–894 (2007) doi:10.1038/sj.jid.5700642.
C. Herkenne, A. Naik, Y. N. Kalia, J. Hadgraft, and R. H. Guy. Ibuprofen transport into and through skin from topical formulations: in vitro–in vivo comparison. J Invest Dermatol. 127:135–142 (2007) doi:10.1038/sj.jid.5700491.
I. Jakasa, M. M. Verberk, M. Esposito, J. D. Bos, and S. Kezic. Altered penetration of polyethylene glycols into uninvolved skin of atopic dermatitis patients. J Invest Dermatol. 127:129–134 (2007) doi:10.1038/sj.jid.5700582.
J. Lademann, A. Ilgevicius, O. Zurbau, H. D. Liess, S. Schanzer, H. J. Weigmann, C. Antoniou, R. V. Pelchrzim, and W. Sterry. Penetration studies of topically applied substances: optical determination of the amount of stratum corneum removed by tape stripping. J Biomed Opt. 11:054026 (2006) doi:10.1117/1.2359466.
B. N'Dri-Stempfer, W. C. Navidi, R. H. Guy, and A. L. Bunge. Improved Bioequivalence Assessment of Topical Dermatological Drug Products Using Dermatopharmacokinetics. Pharm Res (2008) doi:10.1007/s11095-008-9742-9.
B. N'Dri-Stempfer, W. C. Navidi, R. H. Guy, and A. L. Bunge. Optimizing metrics for the assessment of bioequivalence between topical drug products. Pharm Res. 25:1621–1630 (2008) doi:10.1007/s11095-008-9577-4.
C. Pellanda, E. Ottiker, C. Strub, V. Figueiredo, T. Rufli, G. Imanidis, and C. Surber. Topical bioavailability of triamcinolone acetonide: effect of dose and application frequency. Arch Dermatol Res. 298:221–230 (2006) doi:10.1007/s00403-006-0677-x.
C. Pellanda, C. Strub, V. Figueiredo, T. Rufli, G. Imanidis, and C. Surber. Topical bioavailability of triamcinolone acetonide: effect of occlusion. Skin Pharmacol Physiol. 20:50–56 (2007) doi:10.1159/000096172.
S. Wiedersberg, C. S. Leopold, and R. H. Guy. Dermatopharmacokinetics of betamethasone 17-valerate: Influence of formulation viscosity and skin surface cleaning procedure. Eur J Pharm Biopharm (2008) doi:10.1016/j.ejpb.2008.10.001.
S. Wiedersberg, A. Naik, C. S. Leopold, and R. H. Guy. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol (2008) doi:10.1111/j.1365-2133.2008.08757.x.
Y. N. Kalia, I. Alberti, N. Sekkat, C. Curdy, A. Naik, and R. H. Guy. Normalization of stratum corneum barrier function and transepidermal water loss in vivo. Pharm Res. 17:1148–1150 (2000) doi:10.1023/A:1026474200575.
R. L. Andersonand, and J. M. Cassidy. Variation in physical dimensions and chemical composition of human stratum corneum. J Invest Dermatol. 61:30–32 (1973) doi:10.1111/1523-1747.ep12674117.
N. Higo, A. Naik, D. B. Bommannan, R. O. Potts, and R. H. Guy. Validation of reflectance infrared spectroscopy as a quantitative method to measure percutaneous absorption in vivo. Pharm Res. 10:1500–1506 (1993) doi:10.1023/A:1018987612155.
I. Alberti, Y. N. Kalia, A. Naik, J. Bonny, and R. H. Guy. Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum, in vivo. Int J Pharm. 219:11–19 (2001) doi:10.1016/S0378-5173(01)00616-0.
I. Alberti, Y. N. Kalia, A. Naik, J. D. Bonny, and R. H. Guy. In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release. 71:319–327 (2001) doi:10.1016/S0168-3659(01)00244-9.
V. H. Mak, R. O. Potts, and R. H. Guy. Percutaneous penetration enhancement in vivo measured by attenuated total reflectance infrared spectroscopy. Pharm Res. 7:835–841 (1990) doi:10.1023/A:1015960815578.
M. Pellett, A. Watkinson, J. Hadgraft, and K. Brain. Comparison of permeability data from traditional diffusion cells and ATR-FTIR spectroscopy. Part II: determination of diffusional pathlengths in synthetic membranes and human stratum corneum. Int. J. Pharm. 154:217–227 (1997) doi:10.1016/S0378-5173(97)00143-9.
J. C. Tsai, M. J. Cappel, G. L. Flynn, N. D. Weiner, J. Kreuter, and J. J. Ferry. Drug and vehicle deposition from topical applications: use of in vitro mass balance technique with minoxidil solutions. J Pharm Sci. 81:736–743 (1992) doi:10.1002/jps.2600810803.
A. L. Stinchcomb, F. Pirot, G. D. Touraille, A. L. Bunge, and R. H. Guy. Chemical uptake into human stratum corneum in vivo from volatile and non-volatile solvents. Pharm Res. 16:1288–1293 (1999) doi:10.1023/A:1014866001386.
H. S. Carslaw, and J. C. Jaeger. Conduction of Heat in Solids. Oxford University Press, Oxford, 1959, pp. 92–110.
I. Schneider, B. Dobner, R. Neubert, and W. Wohlrab. Evaluation of drug penetration into human skin ex vivo using branched fatty acids and propylene glycol. Int J Pharm. 145:187–196 (1996) doi:10.1016/S0378-5173(96)04768-0.
Acknowledgments
We thank Dr. Sandra Wiedersberg for very helpful discussion and advice.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The concentration of drug in the stratum corneum (SC) during clearance is described by Fick’s second law:
where the concentration at time t app is defined by Eq. 1, the flux of drug from the surface of the stratum corneum is zero, and sink conditions apply at the interface between the SC and viable epidermis:
Equation 3 is solved for these boundary conditions by separation of variables to obtain Eq. 2 (19).
Rights and permissions
About this article
Cite this article
Nicoli, S., Bunge, A.L., Delgado-Charro, M.B. et al. Dermatopharmacokinetics: Factors Influencing Drug Clearance from the Stratum Corneum. Pharm Res 26, 865–871 (2009). https://doi.org/10.1007/s11095-008-9785-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9785-y