Abstract
Purpose
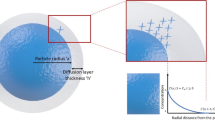
Particle size of a drug is an important factor that affects thermodynamic solubility and oral absorption of drug molecules. Weight fraction of different particle sizes in a polydisperse powder together with Noyes Whitney parameters (diffusion coefficient, solubility, density of the drug, boundary layer thickness and dissolution volume) can be used to predict dissolution and absorption of drug molecules. Such a simulation can be a valuable tool in setting up guidance with regards to dependence of dissolution and absorption on particle size.
Materials and methods
In this note a modified method is reported to predict dissolution of polydisperse drug powder. These use simplified equations developed from a set of differential equations described previously. The idea was to convert all the terms in one single equation which can then be solved by a Matlab program.
Conclusion
Discrepancies not reported earlier have been discussed to get the same results as reported previously.
Similar content being viewed by others
References
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46(1–3):3–26 (2001).
M. V. Varma, S. Khandavilli, Y. Ashokraj, A. Jain, A. Dhanikula, A. Sood, N. S. Thomas, O. Pillai, P. Sharma, R. Gandhi, S. Agrawal, V. Nair, and R. Panchagnula. Biopharmaceutic classification system: a scientific framework for pharmacokinetic optimization in drug research. Curr. Drug Metab. 5(5):375–388 (2004).
R. J. Hintz, and K. C. Johnson. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 51(1):9–17 (1989).
K. C. Johnson. Dissolution and absorption modeling: model expansion to simulate the effects of precipitation, water absorption, longitudinally changing intestinal permeability, and controlled release on drug absorption. Drug Dev. Ind. Pharm. 29(8):833–842 (2003).
K. C. Johnson, and A. C. Swindell. Guidance in the setting of drug particle size specifications to minimize variability in absorption. Pharm. Res. 13(12):1795–1798 (1996).
A. T. Lu, M. E. Frisella, and K. C. Johnson. Dissolution modelling: factors affecting the dissolution rates of polydisperse powders. Pharm. Res. 10(9):1308–1314 (1993).
A. P. Tinke, K. Vanhoutte, R. De Maesschalck, S. Verheyen, and H. De Winter. A new approach in the prediction of the dissolution behavior of suspended particles by means of their particle size distribution. J. Pharm. Biomed. Anal. 39(5):900–907 (2005).
V. B. Patravale, A. A. Date, and R. M. Kulkarni. Nanosuspensions: a promising drug delivery strategy. J. Pharm. Pharmacol. 56(7):827–840 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butcher, J.C., Garg, S., Kim, D. et al. A Modified Approach to Predict Dissolution and Absorption of Polydisperse Powders. Pharm Res 25, 2309–2311 (2008). https://doi.org/10.1007/s11095-008-9630-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9630-3