Abstract
Purpose
To evaluate the behaviour of an oral matrix modified release formulation in the canine gastrointestinal tract, and establish if a mechanical weakness previously observed in clinical studies would have been identified in the dog model.
Materials and Methods
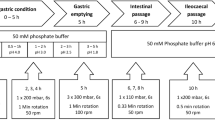
In vitro release profiles were obtained for two modified release matrix tablets containing UK-294,315, designed to release over either 6 (formulation A) or 18 (formulation B) hours. Tablets were labelled with 153samarium and in vivo pharmacoscintigraphy studies were performed in four beagle dogs in the fasted state for both formulations, and following ingestion of an FDA high fat meal for formulation B.
Results
The matrix tablet formulations displayed significantly different in vitro release profiles (F 2 < 50), with time to 80% release for formulation A and B of 406 and 987 min respectively. Complete in vivo disintegration occurred at 339 ± 181 and 229 ± 171 for formulation A and B respectively in the fasted state, and at 207 ± 154 min for formulation B in the fed state, in disagreement with in vitro release.
Conclusion
The fed/fasted dog model would have predicted a lack of physical robustness in the matrix tablet formulation B, however it would not have predicted the clear fed/fasted effects on performance observed previously in man.






Similar content being viewed by others
Notes
4-Amino-5-(4-fluorophenyl)-6,7-dimethoxy-2-[4-(morpholinocarbonyl)-perhydro-1,4-diazepin-1-yl]quinoline
4-amino-5-(4-chlorophenyl)-6,7-dimethoxy-2-[4-(morpholinocarbonyl)-perhydro-1,4-diazepin-1-yl]quinoline
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- AUC(0–8) :
-
Area under the plasma concentration curve from 0–8 h.
- \( {\rm{AUC}}_{{{\left( {0 - \infty } \right)}}} \) :
-
Area under the plasma concentration curve from 0 h extrapolated to infinity.
- CD:
-
Complete tablet disintegration time
- C max :
-
Maximum plasma concentration
- GEC:
-
Gastric emptying complete
- GI:
-
Gastrointestinal
- HPMC:
-
Hydroxypropylmethylcellulose
- ID:
-
Initial tablet disintegration time
- IV:
-
Intravenous
- K el :
-
Elimination rate constant
- MBq:
-
Megabequerel
- MMC:
-
migrating myoelectric complex
- MR:
-
Modified release
- ROI:
-
Region of interest
- SIT:
-
Small intestinal transit time
- Sm:
-
Samarium
- 152Sm:
-
samarium oxide-152
- Tc:
-
Technetium
- T max :
-
Time to reach maximum plasma concentration
- T 50% :
-
Time to 50% of parameter
- T 1/2 :
-
Plasma half life
- 99mTc-DTPA:
-
Technetium-99m-diethylenetriaminepentaacetic acid
References
M. Shameem, N. Katori, N. Aoyagi, and S. Kojima. Oral solid controlled release dosage forms: role of GI-mechanical destructive forces and colonic release in drug absorption under fasted and fed conditions in humans. Pharm. Res. 12(7):1049–1054 (1995).
L. Kalantzi, B. Polentarutti, T. Albery, D. Laitmer, B. Abrahamsson, J. Dressman, and C. Reppas. The delayed dissolution of paracetamol products in the canine fed stomach can be predicted in-vitro but it does not affect the onset of plasma levels. Int. J. Pharm. 296:87–93 (2005).
T. Goto, N. Tanida, T. Yoshinaga, S. Sato, D. J. Ball, I. R. Wilding, E. Kobayashi, and A. Fujimara. Pharmaceutical design of a novel colon-targeted delivery system using two-layer-coated tablets of three different pharmaceutical formulations, supported by clinical evidence in humans. J. Control. Release 97:31–42 (2004).
N. Katori, N. Aoyagi, and Terao. Estimation of agitation intensity in the GI tract in humans and dogs based on in-vitro/in-vivo correlation. Pharm. Res. 12(2):237–243 (1995).
S. Blanquet, E. Zeijdner, E. Beyssac, J. Meunier, S. Denis, R. Havenaar, and M. Alric. A dynamic artificial gastrointestinal system for studying the behaviour of orally administered drug dosage forms under various physiological conditions. Pharm. Res. 21(4):585–591 (2004).
N. Venkatesan, J. Yoshimitsu, Y. Ohashi, Y. Ito, N. Sugioka, N. Shibata, and K. Takada. Pharmacokinetic and pharmacodynamic studies following oral administration of erythropoietin mucoadhesive tablets to beagle dogs. Int. J. Pharm. 310:46–52 (2006).
T. Ishibashi, H. Hatano, M. Kobayashi, M. Mizobe, and H. Yashino. In-vivo drug release behaviour in dogs from a new colon-targeted delivery system. J. Control. Release 57:45–53 (1999).
Y. Wu, A. Loper, E. Landis, L. Hettrick, L. Novak, K. Lynn, C. Chen, K. Thompson, R. Higgins, U. Batra, S. Shelukar, G. Kwei, and D. Storey. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int. J. Pharm. 285:135–146 (2004).
B. K. Kang, J. S. Lee, S. K. Chon, S. Y. Jeong, S. H. Yuk, G. Khang, H. B. Lee, and S. H. Cho. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 274:65–73 (2004).
J. B. Dressman. Comparison of canine and human gastrointestinal physiology. Pharm. Res. 3(3):123–131 (1986).
J. B. Dressman, and K. Yamada. Animal models for oral drug absorption. In P. G. Welling (ed), Pharmaceutical Bioequivalence, Marcel Dekker, New York, 1991, pp. 235–266.
K. Yamada, A. Furuya, M. Akimoto, T. Maki, T. Suwa, and H. Ogata. Evaluation of gastrointestinal transit controlled-beagle dog as a suitable animal model for bioavailability testing of sustained-release acetaminophen dosage form. Int. J. Pharm. 119:1–10 (1995).
M. Akimoto, N. Nagahata, A. Furuya, K. Fukushima, S. Higuchi, and T. Suwa. Gastric pH profiles of beagle dogs and their use as an alternative to human testing. Eur. J. Pharm. Biopharm. 49:99–102 (2000).
C. Y. Lui, G. L. Amidon, R. R. Berardi, D. Fleisher, C. Youngberg, and J. B. Dressman. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J. Pharm. Sci. 75(3):271–274 (1986).
Y. L. He, S. Murby, G. Warhurst, L. Gifford, D. Walker, J. Ayrton, R. Eastmond, and M. Rowland. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly(ethylene glycol) and D-peptides. J. Pharm. Sci. 87(5):626–633 (1998).
W. L. Chiou, H. Y. Jeong, S. M. Chung, and T. C. Wu. Evaluation of using dog as an animal model to study the fraction of oral dose absorbed of 43 drugs in humans. Pharm. Res. 17(2):135–140 (2000).
S. S. Davis, E. A. Wilding, and I. R. Wilding. Gastrointestinal transit of a matrix tablet formulation: comparison of canine and human data. Int. J. Pharm. 94:235–238 (1993).
M. Akimoto, A. Furuya, T. Maki, K. Yamada, T. Suwa, and H. Ogata. Evaluation of sustained-release granules of chlorphenesin carbamate in dogs and humans. Int. J. Pharm. 100:133–142 (1993).
N. Aoyagi, H. Ogata, N. Kaniwa, M. Uchiyama, Y. Yasuda, and Y. Tanioka. Gastric emptying of tablets and granules in humans, dogs, pigs, and stomach-emptying-controlled rabbits. J. Pharm. Sci. 81(12):1170–1174 (1992).
J. H. Meyer, J. Dressman, A. Fink, and G. Amidon. Effect of size and density on canine gastric emptying of non-dogestible solids. Gastroenterology 89:805–813 (1985).
H. N. E. Stevens, C. G. Wilson, P. G. Welling, M. Bakhshaee, J. S. Binns, A. C. Perkins, M. Frier, E. P. Blackshaw, M. W. Frame, D. J. Nichols, M. J. Humphrey, and S. R. Wicks. Evaluation of Pulsincap™ to provide regional delivery of dofetilide to the human GI tract. Int. J. Pharm. 236:27–34 (2002).
I. R. Wilding, S. S. Davis, R. A. Sparrow, J. A. Ziemniak, and D. L. Heald. Pharmacoscintigraphic evaluation of a modified release (Geomatrix®) diltiazem formulation. J. Control. Release 33:89–97 (1995).
K. Kelly, B. O’Mahony, B. Lindsay, T. Jones, T. J. Grattan, A. Rostami-Hodjegan, H. N. E. Stevens, and C. G. Wilson. Comparison of disintegration, gastric emptying, and drug absorption following administration of a new and a conventional paracetamol formulation, using γ scintigraphy. Pharm. Res. 20(10):1668–1673 (2003).
B. Abrahamsson, M. Alpsten, B. Bake, A. Larsson, and J. Sjögren. In-vitro and in-vivo erosion of two different hydrophilic gel matrix tablets. Eur. J. Pharm. Biopharm. 46:69–75 (1998).
M. Kamba, Y. Seta, A. Kusai, and K. Nishimura. Evaluation of the mechanical destructive force in the stomach of dog. Int. J. Pharm. 228:209–217 (2001).
Y. Iwanaga, J. Wen, M. S. Thollander, L. J. Kost, G. M. Thomforde, R. G. Allen, and S. F. Phillips. Scintigraphic measurement of regional gastrointestinal transit in the dog. Am. J. Physiol. 275:G904–G910 (1998).
S. C. Sutton. Companion animal physiology and dosage form performance. Adv. Drug Del. Rev. 56:1383–1398 (2004).
J. D. R. Schulze, E. E. Peters, A. W. Vickers, J. S. Staton, M. D. Coffin, G. E. Parsons, and A. W. Basit. Excipient effects on gastrointestinal transit and drug absorption in beagle dogs. Int. J. Pharm. 300:67–75 (2005).
B. Abrahamsson, T. Albery, A. Eriksson, I. Gustafsson, and M. Sjöberg. Food effects on tablet disintegration. Eur. J. Pharm. Sci. 22:165–172 (2004).
C. S. Cook, L. Zhang, J. Osis, G. L. Schoenhard, and A. Karim. Mechanism of compound- and species-specific food effects of structurally related antiarrhythmic drugs, disopyramide and bidisomide. Pharm. Res. 15(3): 429–433 (1998).
B. Abrahamsson, M. Alpsten, B. Bake, U. E. Johnsson, M. Eriksson-Lepkowska, and A. Larsson. Drug absorption from nifedipine hydrophilic matrix extended-release (ER) tablet-comparison with an osmotic pump tablet and effect of food. J. Control. Release 52:301–310 (1998).
L. Maggi, L. Segale, E. O. Machiste, A. Faucitano, A. Buttafava, and U. Conte. Polymers–gamma ray interaction. Effects of gamma irradiation on modified release drug delivery systems for oral administration. Int. J. Pharm. 269(2):343–351 (2004).
T. Waaler, S. A. Sande, B. W. Müller, and G. Schüller Lisether. The influence of thermal neutron irradiation on the in-vitro characteristics of ASA oral dosage forms. Validation of neutron activation. Eur. J. Pharm. Biopharm. 43:159–164 (1997).
M. Kamba, Y. Seta, A. Kusai, and K. Nishimura. Comparison of the mechanical destructive force in the small intestine of dog and human. Int. J. Pharm. 237:139–149 (2002).
R. A. Hinder, and K. A. Kelly. Canine gastric emptying of solids and liquids. Am. J. Physiol. 233(4):E335–E340 (1977).
J. H. Meyer, E. A. Mayer, D. Jehn, Y. Gu, A. S. Fink, and M. Fried. Gastric processing and emptying of fat. Gastroenterology 90:1176–1187 (1986).
A. Harrison, A. Betts, K. Fenner, K. Beaumont, A. Edgington, S. Roffey, J. Davis, P. Comby, and P. Morgan. Nonlinear oral pharmacokinetics of the α-antagonist 4-Amino-5-(4-fluorophenyl)-6,7-dimethoxy-2-[4-(morpholinocarbonyl)-perhydro-1,4-diazepin-1-yl]quinoline in humans: use of preclinical data to rationalize clinical observations. Drug Metab. Dispos. 32(2):197–204 (2004).
M. Kamba, Y. Seta, A. Kusai, M. Ikeda, and K. Nishimura. A unique dosage form to evaluate the mechanical destructive force in the gastrointestinal tract. Int. J. Pharm. 208:61–70 (2000).
N. Fotaki, M. Symillides, and C. Reppas. In vitro versus canine data for predicting input profiles of isosorbide-5-mononitrate from oral extended release products on a confidence interval basis. Eur. J. Pharm. Sci. 24:115–122 (2005).
M. B. Awang, J. G. Hardy, S. S. Davis, I. R. Wilding, and S. J. Parry. Radiolabeling of pharmaceutical dosage forms by neutron-activation of Sm-152. J. Labeled Compd. Radiopharm. 33(10):941–948 (1993).
Acknowledgments
These studies were funded by Pfizer Global Research and Development, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McInnes, F., Clear, N., Humphrey, M. et al. In Vivo Performance of an Oral MR Matrix Tablet Formulation in the Beagle Dog in the Fed and Fasted State: Assessment of Mechanical Weakness. Pharm Res 25, 1075–1084 (2008). https://doi.org/10.1007/s11095-007-9462-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9462-6