Abstract
Purpose
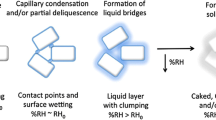
Deliquescence is the process by which a solid undergoes dissolution by sorbing moisture from its surroundings when a characteristic relative humidity, RH0, is reached. For mixtures of two or more deliquescent solids, RH0 will generally be lowered. The goal of this research was to investigate the effect of small amounts of impurities or degradants on RH0 for a model deliquescent pharmaceutical salt.
Materials and Methods
The model salt chosen for this work was ranitidine HCl, which has two polymorphic forms. Moisture sorption profiles for each polymorph and samples of different purities were obtained using a gravimetric water vapor sorption balance.
Results
Polymorphs of a similar purity yielded virtually identical moisture sorption profiles. In contrast, samples containing higher levels of impurities had both enhanced moisture sorption below RH0 and a lowered value of RH0.
Conclusions
It was concluded that small levels of degradants and/or impurities can drastically affect the moisture sorption profile of a deliquescent material, both through affecting the deliquescence relative humidity and by altering the overall interaction of the substance with moisture. Such changes in behavior may have significant effects on both active pharmaceutical ingredient and drug product stability during both processing and storage.





Similar content being viewed by others
References
J. T. Carstensen, A. Gerhardt, T. Morris, and F. Nikfar. Effect of moisture on solid dosage forms—can the Arrhenius equation be used as a predictor. Drug Dev. Ind. Pharm. 16:2267–2281 (1990).
C. Ahlneck and G. Zografi. The molecular-basis of moisture effects on the physical and chemical-stability of drugs in the solid-state. Int. J. Pharm. 62:87–95 (1990).
J. T. Carstensen. Effect of moisture on the stability of solid dosage forms. Drug Dev. Ind. Pharm. 14:1927–1969 (1988).
J. T. Carstensen, F. Attarchi, and X. P. Hou. Decomposition of aspirin in the solid-state in the presence of limited amounts of moisture. J. Pharm. Sci. 74:741–745 (1985).
S. Yoshioka and M. Uchiyama. Kinetics and mechanism of the solid-state decomposition of propantheline bromide. J. Pharm. Sci. 75:92–96 (1986).
S. Yoshioka, T. Shibazaki, and A. Ejima. Stability of solid dosage forms. 1. Hydrolysis of meclofenoxate hydrochloride in the solid-state. Chem. Pharm. Bull. 30:3734–3741 (1982).
R. Teraoka, M. Otsuka, and Y. Matsuda. Effects of temperature and relative-humidity on the solid-state chemical-stability of ranitidine hydrochloride. J. Pharm. Sci. 82:601–604 (1993).
K. Uzunarslan and J. Akbuga. The effect of moisture on the physical characteristics of ranitidine hydrochloride tablets prepared by different binders and techniques. Drug Dev. Ind. Pharm. 17:1067–1081 (1991).
K. Uzunarslan and J. Akbuga. Moisture adsorption and effect of adsorbed water on the powder and compaction properties of ranitidine hydrochloride. Pharmazie 46:273–275 (1991).
G. Zografi. States of water associated with solids. Drug Dev. Ind. Pharm. 14:1905–1926 (1988).
K. Danjo, H. Kato, A. Otsuka, and T. Wakimoto. Influence of moisture adsorption on volume shrinkage and diametral tensile-strength of sucrose tablets. Chem. Pharm. Bull. 41:2147–2150 (1993).
M. Otsuka and Y. Matsuda. The effect of humidity on hydration kinetics of mixtures of nitrofurantoin anhydride and diluents. Chem. Pharm. Bull. 42:156–159 (1994).
A. K. Salameh and L. S. Taylor. Deliquescence in binary mixtures. Pharm. Res. 22:318–324 (2005).
L. Van Campen, G. Zografi, and J. T. Carstensen. An approach to the evaluation of hygroscopicity for pharmaceutical solids. Int. J. Pharm. 5:1–18 (1980).
M. J. Kontny and G. Zografi. Sorption of water by solids. Drugs Pharm. Sci. 70:387–418 (1995).
L. Van Campen, G. L. Amidon, and G. Zografi. Moisture sorption kinetics for water-soluble substances. 1. Theoretical considerations of heat-transport control. J. Pharm. Sci. 72:1381–1388 (1983).
S. D. Brooks, M. E. Wise, M. Cushing, and M. A. Tolbert. Deliquescence behavior of organic/ammonium sulfate aerosol. Geophys. Res. Lett. 29:1917–1920 (2002).
S. L. Clegg, J. H. Seinfeld, and P. Brimblecombe. Thermodynamic modelling of aqueous aerosols containing electrolytes and dissolved organic compounds. J. Aerosol Sci. 32:713–738 (2001).
J. S. Lin and A. Tabazadeh. The effect of nitric acid uptake on the deliquescence and efflorescence of binary ammoniated salts in the upper troposphere. Geophys. Res. Lett. 29:1488 (2002).
C. Marcolli, B. P. Luo, and T. Peter. Mixing of the organic aerosol fractions: liquids as the thermodynamically stable phases. J. Phys. Chem. A 108:2216–2224 (2004).
I. N. Tang, H. R. Munkelwitz, and J. G. Davis. Aerosol growth studies—IV: phase transformation of mixed salt aerosols in a moist atmosphere. J. Aerosol Sci. 9:505–511 (1978).
N. Beaulieu, P. M. Lacroix, R. W. Sears, and E. G. Lovering. High-performance liquid-chromatographic methods for the determination of ranitidine and related substances in raw-materials and tablets. J. Pharm. Sci. 77:889–892 (1988).
M. B. Evans, P. A. Haywood, D. Johnson, M. Martinsmith, G. Munro, and J. C. Wahlich. Chromatographic methods for determining the identity, strength and purity of ranitidine hydrochloride both in the drug substance and its dosage forms—an exercise in method selection, development, definition and validation. J. Pharm. Biomed. Anal. 7:1–22 (1989).
P. A. Haywood, M. Martinsmith, T. J. Cholerton, and M. B. Evans. Isolation and identification of the hydrolytic degradation products of ranitidine hydrochloride. J. Chem. Soc., Perkin Trans. 1:951–954 (1987).
M. A. Kelly, K. D. Altria, C. Grace, and B. J. Clark. Optimisation, validation and application of a capillary electrophoresis method for the determination of ranitidine hydrochloride and related substances. J. Chromatogr. A 798:297–306 (1998).
H. Nyqvist. Saturated salt solutions for maintaining specified relative humidities. Int. J. Pharm. Technol. Prod. Manuf. 4:47–48 (1983).
V. Wu, T. Rades, and D. J. Saville. Stability of polymorphic forms of ranitidine hydrochloride. Pharmazie 55:508–512 (2000).
A. K. Salameh, L. J. Mauer, and L. S. Taylor. Deliquescence lowering in food ingredient mixtures. J. Food Sci. 71:E10–E16 (2006).
I. N. Tang and K. H. Fung. Hydration and Raman scattering studies of levitated microparticles: Ba(NO(3))(2), Sr(NO3)(2), and Ca(NO3)(2). J. Chem. Phys. 106:1653–1660 (1997).
J. T. Carstensen and M. K. Franchini. Isoenergetic polymorphs. Drug Dev. Ind. Pharm. 21:523–536 (1995).
B. C. Hancock and G. Zografi. Effects of solid-state processing on water vapor sorption by aspirin. J. Pharm. Sci. 85:246–248 (1996).
R. Saklatvala, P. G. Royall, and D. Q. M. Craig. The detection of amorphous material in a nominally crystalline drug using modulated temperature DSC—a case study. Int. J. Pharm. 192:55–62 (1999).
S. E. Dilworth, G. Buckton, S. Gaisford, and R. Ramos. Approaches to determine the enthalpy of crystallisation, and amorphous content, of lactose from isothermal calorimetric data. Int. J. Pharm. 284:83–94 (2004).
G. H. Ward and R. K. Schultz. Process-induced crystallinity changes in albuterol sulfate and its effect on powder physical stability. Pharm. Res. 12:773–779 (1995).
A. Saleki-Gerhardt, C. Ahlneck, and G. Zografi. Assessment of Disorder in Crystalline Solids. Int. J. Pharm. 101:237–247 (1994).
T. Luty and C. J. Eckhardt. General theoretical concepts for solid-state reactions—quantitative formulation of the reaction cavity, steric compression, and reaction-induced stress using an elastic multipole representation of chemical pressure. J. Am. Chem. Soc. 117:2441–2452 (1995).
S. R. Byrn, R. R. Pfeifffer, and J. G. Stowell. Solid-State Chemistry of Drugs, SSCI, West Lafayette, Indiana, 1999.
S. Ghosal and J. C. Hemminger. Surface adsorbed water on NaCl and its effect on nitric acid reactivity with NaCl powders. J. Phys. Chem. B 108:14102–14108 (2004).
M. Luna, F. Rieutord, N. A. Melman, Q. Dai, and M. Salmeron. Adsorption of water on alkali halide surfaces studied by scanning polarization force microscopy. J. Phys. Chem. A 102:6793–6800 (1998).
Acknowledgments
The authors acknowledge AstraZeneca R&D Lund, Sweden for funding this research. Dr Kjell Järring and Dr Frans Langkilde are thanked for helpful comments. Dr. Dan Smith is thanked for providing use of the Agilent 1100 HPLC system. Dr. Karl Wood is thanked for his discussions regarding the LC/MS results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerrieri, P., Salameh, A.K. & Taylor, L.S. Effect of Small Levels of Impurities on the Water Vapor Sorption Behavior of Ranitidine HCl. Pharm Res 24, 147–156 (2007). https://doi.org/10.1007/s11095-006-9134-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9134-y