Abstract
Purpose
Use RH-perfusion microcalorimetry and other analytical techniques to measure the interactions between water vapor and amorphous pharmaceutical solids; use these measurements and a mathematical model to provide a mechanistic understanding of observed calorimetric events.
Materials
Isothermal microcalorimetry was used to characterize interactions of water vapor with a model amorphous system, spray-dried raffinose. Differential scanning calorimetry was used to measure glass transition temperature, T g. High-sensitivity differential scanning calorimetry was used to measure enthalpy relaxation. X-ray powder diffraction (XRPD) was used to confirm that the spray-dried samples were amorphous. Scanning electron microscopy (SEM) was used to examine particle morphology. Gravimetric vapor sorption was used to measure moisture sorption isotherms. Thermogravimetric analysis (TGA) was used to measure loss on drying.
Results
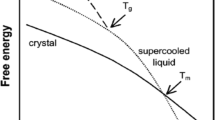
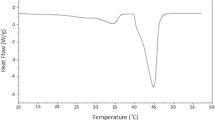
A moisture-induced thermal activity trace (MITAT) provides a rapid measure of the dependence of molecular mobility on moisture content at a given storage temperature. At some relative humidity threshold, RHm, the MITAT exhibits a dramatic increase in the calorimetric rate of heat flux. Simulations using calorimetric data indicate that this thermal event is a consequence of enthalpy relaxation.
Conclusions
RH-perfusion microcalorimetry is a useful tool to determine the onset of moisture-induced physical instability of glassy pharmaceuticals and could find a broad application to determine appropriate storage conditions to ensure long-term physical stability. Remarkably, thermal events measured on practical laboratory timescales (hours to days) are relevant to the stability of amorphous materials on much longer, pharmaceutically relevant timescales (years). The mechanistic understanding of these observations in terms of enthalpy relaxation has added further value to the use of RH-perfusion calorimetry as a rapid means to characterize the molecular mobility of amorphous solids.













Similar content being viewed by others
References
G. Van den Mooter, P. Augustijns, and R. Kinget. Stability prediction of amorphous benzodiazepines by calculation of the mean relaxation time constant using the Williams–Watts decay function. Eur. J. Pharm. Biopharm. 48(1):43–48 (1999).
S. P. Duddu, G. Zhang, and P. R. Dal Monte. The relationship between protein aggregation and molecular mobility below the glass transition temperature of lyophilized formulations containing a monoclonal antibody. Pharm. Res. 14(5):596–600 (1997).
R. A. Shmeis, Z. Wang, and S. L. Krill. A mechanistic investigation of an amorphous pharmaceutical and its solid dispersions, part I: A comparative analysis by thermally stimulated depolarization current and differential scanning calorimetry. Pharm. Res. 21:2025–2030 (2004).
S. Yoshioka, S. Tajima, Y. Aso, and S. Kojima. Inactivation and aggregation of beta-galactosidase in lyophilized formulation described by Kohlrausch–Williams–Watts stretched exponential function. Pharm. Res. 20(10):1655–1660 (2003).
J. Liu, D. R. Rigsbee, C. Stotz, and M. J. Pikal. Dynamics of pharmaceutical amorphous solids: the study of enthalpy relaxation by isothermal microcalorimetry. J. Pharm. Sci. 91:1853–1862 (2002).
V. Andronis and G. Zografi. The molecular mobility of supercooled amorphous indomethacin as a function of temperature and relative humidity. Pharm. Res. 15(6):835–842 (1998).
S. P. Duddu and T. D. Sokoloski. Dielectric analysis in the characterization of amorphous pharmaceutical solids. 1. Molecular mobility in poly(vinylpyrrolidone)—water systems in the glassy state. J. Pharm. Sci. 84:773–776 (1995).
S. Yoshioka, Y. Aso, and S. Kojima. Temperature- and glass transition temperature-dependence of bimolecular reaction rates in lyophilized formulations described by the Adam–Gibbs–Vogel equation. J. Pharm. Sci. 93(4):1062–1069 (2004).
H. Hu and C. T. Sun. The equivalence of moisture and temperature in physical aging of polymeric composites. J. Compos. Mater. 37:913–928 (2003).
B. Borde, H. Bizot, G. Vigier, and A. Buléon. Calorimetric analysis of the structural relaxation in partially hydrated amorphous polysaccharides. I. Glass transition and fragility. Carbohydr. Polym. 48:83–96 (2002).
D. Lechuga-Ballesteros, A. Bakri, and D. P. Miller. Microcalorimetric measurement of the interactions between water vapor and amorphous pharmaceutical solids. Pharm. Res. 20(2):308–318 (2003).
B. Makower and W. B. Dye. Equilibrium moisture content and crystallization of amorphous sucrose and glucose. J. Agric. Food Chem. 4:72–77 (1956).
H. Binder, B. Kohlstrunk, and H. H. Heerklotz. Hydration and lyotropic melting of amphiphilic molecules: a thermodynamic study using humidity titration calorimetry. J. Colloid Interface Sci. 220(2):235–249 (1999).
V. P. Lehto and E. Laine. Simultaneous determination of the heat and the quantity of vapor sorption using a novel microcalorimetric method. Pharm. Res. 6:701–706 (2000).
M. Pudipeddi, T. D. Sokoloski, S. P. Duddu, and J. T. Carstensen. Quantitative characterization of adsorption isotherms using isothermal microcalorimetry. J. Pharm. Sci. 85(4):381–386 (1996).
L. Greenspan. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sec., A. 81:89–102 (1977).
A. Bakri. Design, testing and pharmaceutical applications of a gas pressure controller device for solid-gas microcalorimetric titration. In ThermoMetric Application Note 22021, Thermometric AB, Sweden, 4 (1993).
K. Chatterjee, E. Y. Shalaev, and R. Suryanarayanan. Partially crystalline systems in lyophilization: I. Use of ternary state diagrams to determine extent of crystallization of bulking agent. J. Pharm. Sci. 94(4):798–808 (2005).
K. Kajiwara, F. Franks, P. Echlin, and A. L. Greer. Structural and dynamic properties of crystalline and amorphous phases in raffinose-water mixtures. Pharm. Res. 16:1441–1448 (1999).
A. Saleki-Gerhardt, J. G. Stowell, S. R. Byrn, and G. Zografi. Hydration and dehydration of crystalline and amorphous forms of raffinose. J Pharm. Sci. 84(3):318–323 (1995).
G. E. Downton, J. L. Flores-Luna, and C. J. King. Mechanism of stickiness in hygroscopic,. amorphous powders. Ind. Eng. Chem. Fundam. 21:447–451 (1982).
M. E. Lazar, A. H. Brown, G. S. Smith, F. F. Wong, and F. E. Lindquist. Experimental production of tomato powder by spray drying. Food Technol. 3:129–134 (1956).
D. A. Wallack and C. J. King. Sticking and agglomeration of hygroscopic amorphous carbohydrate and food powders. Biotechnol. Prog. 4(1):31–35 (1988).
Y. Frenkel. Viscous flow of crystalline bodies under the action of surface tension. J. Phys. (USSR). 9(5):385–391 (1945).
M. Peleg and C. H. Mannheim. The mechanism of caking of powdered onion. J. Food Process. Preserv. 1:3–11 (1977).
B. C. Hancock, C. R. Dalton, M. J. Pikal, and S. L. Shamblin. A pragmatic test of a simple calorimetric method for determining the fragility of some amorphous pharmaceutical materials. Pharm. Res. 15(5):762–767 (1998).
I. M. Hodge. Adam–Gibbs formulation of enthalpy relaxation near the glass transition. J. Res. Natl. Inst. Sci. Technol. 102:195–205 (1997).
C. A. Angell, K. L. Ngai, G. B. McKenna, P. F. McMillan, and S. W. Martin. Relaxation in glassforming liquids and amorphous solids. J. Appl. Phys. 88:3113–3157 (2000).
C. T. Moynihan. Structural relaxation and the glass transition. Rev. Miner. 32:1–19 (1995).
L. C. E. Struik. Physical Aging in Amorphous Polymers and other Materials, Elsevier, Amsterdam, 1978.
A. Q. Tool. Relations between inelastic deformability and thermal expansion of glass in its annealing range. J. Am. Ceram. Soc. 29:240 (1946).
O. S. Narayanaswamy. A model of structural relaxation in glass. J. Am. Ceram. Soc. 54(10):491 (1971).
G. B. McKenna. On the physics required for the prediction of long–term performance of polymers and their composites. J. Res. Natl Inst. Stand. Technol. 99:169–189 (1994).
F. Kohlrausch. Pogg. Ann. Phys. 199:352 (1863).
G. Williams and D. C. Watts. Non-symmetrical dielectric relaxation behavior arising from a simple decay function. Trans. Faraday Soc. 66:80–85 (1970).
J. M. G. Cowie, S. Harris, and I. J. McEwen. Physical aging in poly(vinyl acetate) 2. Relative rates of volume and enthalpy relaxation. Macromolecules. 31:2611–2615 (1998).
B. C. Hancock, S. L. Shamblin, and G. Zografi. The molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm. Res. 12(6):799–806 (1995).
S. Yoshioka, Y. Aso, and S. Kojima. Usefulness of the Kohlrausch–Williams–Watts stretched exponential function to describe protein aggregation in lyophilized formulations and the temperature dependence near the glass transition temperature. Pharm. Res. 18(3):256–260 (2001).
R. Böhmer, K. L. Ngai, C. A. Angell, and D. J. Plazek. Non-exponential relaxations in strong and fragile glass-formers. J. Chem. Phys. 99(5):4201–4209 (1993).
H. L. Hampsch, J. Yang, G. K. Wong, and J. M. Torkelson. Macromolecules. 23:3640–3647 (1990).
K. Kawakami and M. J. Pikal. Calorimetric investigation of the structural relaxation of amorphous materials: evaluating validity of the methodologies. J. Pharm. Sci. 94(5):948–965 (2005).
I. M. Hodge and A. R. Berens. Effects of annealing and prior history on enthalpy relaxation in glassy polymers 2. Mathematical modeling. Macromolecules 15:762–770 (1982).
J. N. Hay and M. J. Jenkins. Simulation of the glass transition. J. Therm. Anal. Calorim. 56:1005–1010 (1999).
S. L. Shamblin, B. C. Hancock, Y. Dupuis, and M. J. Pikal. Interpretation of relaxation time constants for amorphous pharmaceutical systems. J. Pharm. Sci.. 89(3):417–427 (2000).
D. J. Plazek and C. A. Bero. Precise glass temperatures. J. Phys., Condens. Matter. 15:S789–S802 (2003).
M. Yoshioka, B. C. Hancock, and G. Zografi. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J. Pharm. Sci. 83(12):1700–1705 (1994).
J. T. Carstensen and K. Van Scoik. Amorphous-to-crystalline transformation of sucrose. Pharm. Res. 7(12):1278–1281 (1990).
Acknowledgments
The authors gratefully acknowledge our colleagues from Nektar Therapeutics for helpful discussions and insight provided throughout the course of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, D.P., Lechuga-Ballesteros, D. Rapid Assessment of the Structural Relaxation Behavior of Amorphous Pharmaceutical Solids: Effect of Residual Water on Molecular Mobility. Pharm Res 23, 2291–2305 (2006). https://doi.org/10.1007/s11095-006-9095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9095-1