Objective
The objective was to investigate pig ear skin as a surrogate for human skin in the assessment of topical drug bioavailability by sequential tape-stripping of the stratum corneum (SC). The potential benefits of ex vivo investigations are manifold: ethical approval is not required, multiple replicate experiments are more easily performed, and toxic compounds can be evaluated.
Materials and Methods
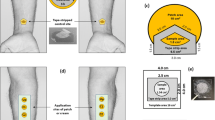
Ex vivo experiments on isolated pig ears were compared with in vivo studies in human volunteers. Four formulations, comprising the model drug, ibuprofen, in different propylene glycol (PG)-water mixtures (25:75, 50:50, 75:25 and 100:0), were compared.
Results
Derived dermatopharmacokinetic parameters characterizing the diffusion and partitioning of the drug in the SC ex vivo were consistent with those in vivo following a 30-minute application period. Further, the non-steady-state ex vivo results could be used to predict the in vivo concentration profile of the drug across the SC when a formulation was administered for 3 h (i.e., close to steady-state).
Conclusions
Taken together, the results obtained suggest that pig ear skin ex vivo has promise as a tool for topical formulation evaluation and optimization.



Similar content being viewed by others
References
I. Alberti, Y. N. Kalia, A. Naik, J. D. Bonny, and R. H. Guy. Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum in vivo. Int. J. Pharm. 219:11–19 (2001).
I. Alberti, Y. N. Kalia, A. Naik, and R. H. Guy. Assessment and prediction of the cutaneous bioavailability of topical terbinafine in vivo in man. Pharm. Res. 18:1472–1475 (2001).
R. L. Anderson and J. M. Cassidy. Variations in physical dimensions and chemical composition of human stratum corneum. J. Invest. Dermatol. 61:30–32 (1973).
D. P. Conner: Differences in DPK Methods. http://www.fda.gov/ohrms/dockets/ac/01/slides/3804s2_05_conner/index.htm, Advisory Committee for Pharmaceutical Sciences Meeting, Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA), Rockville, Maryland, November 29, 2001.
T. J. Franz. Study #1, Avita Gel 0.025% vs Retin-A Gel 0.025%, Advisory committee for pharmaceutical sciences meeting, Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA), Rockville, Maryland, November 29, 2001.
Y. N. Kalia, F. Pirot, and R. H. Guy. Homogeneous transport in a heterogeneous membrane: water diffusion across human stratum corneum in vivo. Biophys. J. 71:2692–2700 (1996).
Y. N. Kalia, I. Alberti, A. Naik, and R. H. Guy. Assessment of topical bioavailability in vivo: the importance of stratum corneum thickness. Skin Pharmacol. 14:82–86 (2001).
A. L. Krogstad, P. A. Jansson, P. Gisslen, and P. Lonnroth. Microdialysis methodology for the measurement of dermal interstitial fluid in humans. Br. J. Dermatol. 134:1005–1012 (1996).
A. W. McKenzie and R. B. Stoughton. Method of comparing percutaneous absorption of steroids. Arch. Dermatol. 86:608–610 (1962).
R. Panchagnula, P. S. Salve, N. S. Thomas, A. K. Jain, and P. Ramarao. Transdermal delivery of naloxone: effect of water, propylene glycol, ethanol and their binary combinations on permeation through rat skin. Int. J. Pharm. 219:95–105 (2001).
L. K. Pershing, B. S. Silver, G. G. Krueger, V. P. Shah, and J. P. Skelley. Feasibility of measuring the bioavailability of topical betamethasone dipropionate in commercial formulations using drug content in skin and a skin blanching bioassay. Pharm. Res. 9:45–51 (1992).
L. K. Pershing, L. D. Lambert, V. P. Shah, and S. Y. Lam. Variability and correlation of chromameter and tape-stripping methods with the visual skin blanching assay in the quantitative assessment of topical 0.05% betamethasone dipropionate bioavailability in humans. Int. J. Pharm. 86:201–210 (1992).
L. K. Pershing. Bioequivalence assessment of three 0.025% tretinoin gel products: dermatopharmacokinetic vs clinical trial methods, advisory committee for pharmaceutical sciences meeting, Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA), Rockville, Maryland, November 29, 2001.
N. Sekkat, Y. N. Kalia, and R. H. Guy. Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. J. Pharm. Sci. 91:2376–2381 (2002).
N. Sekkat and R. H. Guy. Biological models to study skin permeation. In: B. Testa, H. Van de Waterbeemd, G. Folkers, and R. H. Guy (eds.), Pharmacokinetic Optimisation in Drug Research, Wiley-VCH and VHCA, Zürich, 2001, pp. 155–172.
V. P. Shah, G. L. Flynn, R. H. Guy, H. I. Maibach, H. Schaefer, J. P. Skelly, R. C. Wester, and A. Yacobi. In vivo percutaneous penetration/absorption. Pharm. Res. 8:1071–1075 (1991).
S. Shah, D. Hare, S. V. Dighe, and R. L. Williams. Bioequivalence of topical dermatological products. In: V. P. Shah and H. I. Maibach (eds.), Topical Drug Bioavailability, Bioequivalence and Penetration, Plenum, New York, 1993, pp. 393–413.
V. P. Shah. Challenges in evaluating bioequivalence of dermatological drug products. In: C. Surber, P. Elsner, and A. J. Birhcer (eds.), Exogenous Dermatology. Curr Prob Dermatol, Karger, Basel, 1995, pp. 152–157.
V. P. Shah. Topical Dermatological Drug Product NDAs and ANDAs—In Vivo Bioavailability, Bioequivalence, In Vitro Release and Associated Studies. US Dept. Of Health and Human Services, Rockville, 1998, pp. 1–19.
I. Tegeder, U. Muth-Selbach, J. Lötsch, G. Rüsing, R. Oelkers, K. Brune, S. Meller, G. R. Kelm, F. Sörgel, and G. Geisslinger. Application of microdialysis for the determination of muscle and subcutaneous tissue concentrations after oral and topical ibuprofen administration. Clin. Pharmacol. Ther. 65:357–368 (1999).
L. Trottet, C. Merly, M. Mirza, J. Hadgraft, and A. F. Davis. Effect of finite doses of propylene glycol on enhancement of in vitro percutaneous permeation of loperamide hydrochloride. Int. J. Pharm. 274:213–219 (2004).
Acknowledgments
We thank Leo Pharmaceutical Products (Denmark) for financial support. We are indebted to the following individuals for stimulating discussion, suggestions, comments and criticism of the work performed: Drs. A. Jorgensen, E. Didriksen, A. Fullerton, V. Shah and, in particular, Professor Annette Bunge from the Colorado School of Mines (Golden, CO, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herkenne, C., Naik, A., Kalia, Y.N. et al. Pig Ear Skin ex Vivo as a Model for in Vivo Dermatopharmacokinetic Studies in Man. Pharm Res 23, 1850–1856 (2006). https://doi.org/10.1007/s11095-006-9011-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9011-8