No Heading
Purpose.
To study i) phase transitions in raffinose solution in the frozen state and during freeze-drying and ii) evaluate the impact of raffinose crystallization on the recovery of protein activity in reconstituted lyophiles.
Methods.
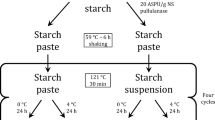
X-ray powder diffractometry (XRD) and differential scanning calorimetry (DSC) were used to study the frozen aqueous solutions of raffinose pentahydrate. Phase transitions during primary and secondary drying were monitored by simulating the entire freeze-drying process, in situ, in the sample chamber of the diffractometer. The activity of lactate dehydrogenase (LDH) in reconstituted lyophiles was determined spectrophotometrically.
Results.
Raffinose formed a kinetically stable amorphous freeze-concentrated phase when aqueous solutions were frozen at different cooling rates. When these solutions were subjected to primary drying without annealing, raffinose remained amorphous. Raffinose crystallized as the pentahydrate when the solutions were annealed at a shelf temperature of −10°C. Primary drying of these annealed systems resulted in the dehydration of raffinose pentahydrate to an amorphous phase. The phase separation of the protein from the amorphous raffinose in these two systems during freeze-drying resulted in a significant reduction in the recovery of LDH activity, even though the lyophile was amorphous.
Conclusions.
Annealing of frozen aqueous raffinose solutions can result in solute crystallization, possibly as the pentahydrate. The crystalline pentahydrate dehydrates during primary drying to yield an amorphous lyophile. Raffinose crystallization during freeze-drying is accompanied by a significant loss of protein activity.
Similar content being viewed by others
References
1. M. J. Pikal. Mechanisms of protein stabilization during freeze-drying and storage: the relative importance of thermodynamic stabilization and glassy state relaxation dynamics. Drugs Pharm. Sci. 96:161–198 (1999).
2. A. Saleki-Gerhardt and G. Zografi. Non-isothermal and isothermal crystallization of sucrose from the amorphous state. Pharm. Res. 11:1166–1173 (1994).
3. O. S. McGarvey, V. L. Kett, and D. Q. M. Craig. An investigation into the crystallization of α,α-trehalose from the amorphous state. J. Phys. Chem. B 107:6614–6620 (2003).
4. A. Saleki-Gerhardt, J. G. Stowell, S. R. Byrn, and G. Zografi. Hydration and dehydration of crystalline and amorphous forms of raffinose. J. Pharm. Sci. 84:318–323 (1995).
5. K. Kajiwara, A. Motegi, M. Sugie, F. Franks, S. Munekawa, T. Igarashi, and A. Kishi. Studies on raffinose hydrates. In H. Levine (ed.), Amorphous Food and Pharmaceutical Systems, Royal Society of Chemistry, Cambridge, 2002, pp. 121–130.
6. K. Kajiwara, F. Franks, P. Echlin, and A. L. Greer. Structural and dynamic properties of crystalline and amorphous phases in raffinose-water mixtures. Pharm. Res. 16:1441–1448 (1999).
7. P. Davidson and W. Q. Sun. Effect of sucrose/raffinose mass ratios on the stability of co-lyophilized protein during storage above the Tg. Pharm. Res. 18:474–479 (2001).
8. A. Pyne, K. Chatterjee, and R. Suryanarayanan. Dehydration of crystalline buffer salt during primary drying. Pharm. Res. 20:802–803 (2003).
9. E. H. Hungerford and A. R. Nees. Raffinose preparation and properties. Ind. Eng. Chem 26:462–464 (1934).
10. J. F. Carpenter, B. S. Chang, W. Garzon-Rodriguez, and T. W. Randolph. Rational design of stable lyophilized protein formulations: theory and practice. In J.F. Carpenter and M.C. Manning (eds.), Pharmaceutical Biotechnology, Volume 13, Kluwer Academic/Plenum Publishers, New York, 2002, p. 203.
11. H. U. Bergmeyer and E. Bernt. Lactate dehydrogenase. In H.U. Bergmeyer (ed.), Methods of Enzymatic Analysis, Academic Press, London, 1965, pp. 574–579.
12. T. J. Anchordoquy, K. Izutsu, T. W. Randolph, and J. F. Carpenter. Maintenance of quaternary structure in the frozen state stabilizes lactate dehydrogenase during freeze-drying. Arch. Biochem. Biophys. 390:35–41 (2000).
13. A. Pyne, R. Surana, and R. Suryanarayanan. Crystallization of mannitol below Tg’R during freeze-drying in binary and ternary aqueous systems. Pharm. Res. 19:901–908 (2002).
14. J. A. Searles, J. F. Carpenter, and T. W. Randolph. Annealing to optimize the primary drying rate, reduce freezing-induced drying rate heterogeneity, and determine Tg’R in pharmaceutical lyophilization. J. Pharm. Sci. 90:872–887 (2001).
15. J. W. Mullin and C. L. Leci. Nucleation characteristics of aqueous citric acid solutions. J. Crys. Growth. 5:75–76 (1969).
16. D. Turnbull and J. C. Fisher. Rate of nucleation in condensed systems. J. Chem. Phys. 17:71–73 (1949).
17. K. Kajiwara and F. Franks. Crystalline and amorphous phases in the binary system water-raffinose. J. Chem. Soc. Far. Trans. 93:1779–1783 (1997).
18. D. Turnbull. Correlation of liquid-solid interfacial energies calculated from supercooling of small droplets. J. Chem. Phys. 18:769 (1950).
19. A. Pyne and R. Suryanarayanan. The effect of additives on the crystallization of cefazolin sodium during freeze drying. Pharm. Res. 20:283–291 (2003).
20. S. N. Timasheff. Water as ligand: preferential binding and exclusion of denaturants in protein unfolding. Biochem 31:9857–9864 (1992).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, K., Shalaev, E. & Suryanarayanan, R. Raffinose Crystallization During Freeze-Drying and Its Impact on Recovery of Protein Activity. Pharm Res 22, 303–309 (2005). https://doi.org/10.1007/s11095-004-1198-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1198-y