No Heading
Purpose.
The main objective of the study was to determine the ability of microthermal analysis (μTA) to assess the crystallinity of the drug in two noncommercial pharmaceutical solid dispersions.
Methods.
Pure substances, physical mixes, and solid dispersions were analyzed by μTA. The thermal values obtained by μTA were compared with data obtained by more conventional techniques like differential scanning calorimetry.
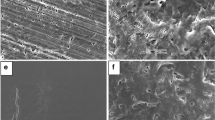
Results.
μTA was able to detect the drug in the waxy matrix. The technique was capable of showing that relatively large amorphous drug domains (up to 70 μm) are formed during the solidification of the solid dispersion. These amorphous domains are thermodynamically unstable and can crystallize upon aging.
Conclusions.
μTA was successfully applied to the physical characterization of solid dispersions. Local thermal analysis (LTA) offers a unique way to probe a selected area on the surface of the sample. It is capable of melting the drug locally without melting the lower melting point excipient. These results gave an understanding of the poor dissolution performance of these solid dispersions upon aging.
Similar content being viewed by others
Abbreviations
- LTA:
-
local thermal analysis
- MDSC:
-
modulated differential scanning calorimetry
- PEG:
-
polyethylene glycol
- PXRD:
-
powder X-ray diffraction
- TPGS:
-
d-α tocopheryl polyethylene glycol 1000 succinate
- μTA:
-
microthermal analysis
References
1. A. T. M. Serajuddin. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent break-throughs. J. Pharm. Sci. 88:1058–1066 (1999).
2. C. Leuner and J. Dressman. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 50:47–60 (2000).
3. C. Y. Perng, A. S. Kearney, K. Patel, N. R. Palepu, and G. Zuber. Investigation of formulation approaches to improve the dissolution of SB-210661, a poorly water soluble 5-lipoxygenase inhibitor. Int. J. Pharm. 176:31–38 (1998).
4. A. Hammiche, M. Reading, H. M. Pollock, M. Song, and D. J. Hourston. Localized thermal analysis using a miniaturized resistive probe. Rev. Sci. Instrum. 67:4268–4274 (1996).
5. D. M. Price, M. Reading, A. Hammiche, and H. M. Pollock. Micro-thermal analysis: Scanning thermal microscopy and localized thermal analysis. Int. J. Pharm. 192:85–96 (1999).
6. R. Häßler and E. Z. Mühlen. An introduction to μTA and its application to the study of interfaces. Thermochim. Acta 361:113–120 (2000).
7. D. Q. M. Craig, V. L. Kett, C. S. Andrews, and P. G. Royall. Pharmaceutical applications of micro-thermal analysis. J. Pharm. Sci. 91:1201–1213 (2002).
8. P. G. Royall, D. Q. M. Craig, D. M. Price, M. Reading, and T. J. Lever. An investigation into the use of micro-thermal analysis for the characterisation of an HPMC tablet formulation. Int. J. Pharm. 192:97–103 (1999).
9. G. H. W. Sanders, C. J. Roberts, A. Danesh, A. J. Murray, D. M. Price, M. C. Davies, S. J. B. Tendler, and M. J. Wilkins. Discrimination of polymorphic forms of a drug product by localized thermal analysis. J. Microsc. 198:77–81 (2000).
10. L. Bond, S. Allen, M. C. Davies, C. J. Roberts, A. P. Shivji, S. J. B. Tendler, P. M. Williams, and J. Zhang. Differential scanning calorimetry and scanning thermal microscopy analysis of pharmaceutical materials. Int. J. Pharm. 243:71–82 (2002).
11. C. T. Moynihan, A. J. Easteal, and J.Wilder. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 78:2673–2677 (1974).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galop, M. Study of Pharmaceutical Solid Dispersions by Microthermal Analysis. Pharm Res 22, 293–302 (2005). https://doi.org/10.1007/s11095-004-1197-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1197-z