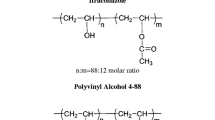
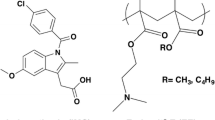
To increase the performance of indomethacin (IND) by enhancing its water solubility and promoting its therapeutic efficiency, a ternary physically stable co-amorphous system of this poorly soluble drug with paracetamol and polyvinylpyrrolidone (PVP K30) was prepared. The effect of PVP on molecular interactions was also evaluated. Binary solid dispersions (IND:paracetamol) were prepared by a kneading method. Then, the PVP as a third component was added at different weight ratios (w/w). The generated systems were characterized by x-ray diffraction, Fourier transform infrared (FTIR) spectroscopy, scanning electronic microscopy, and 13C NMR spectroscopy. As evidenced by FTIR spectroscopy and 13C NMR investigations, the PVP interacted with paracetamol and IND through hydrogen bonds. A physical stability study under high stress conditions at relative humidity = 75% and T = 40°C for 3 months showed the ability of PVP to stabilize amorphous drug molecules. This has significantly improved the water solubility of IND at T = 37°C and pH = 7.0 in comparison with the binary solid dispersion (IND:paracetamol) or the pure state of the drug. The synergistic effect that PVP provides to the binary mixture of IND and paracetamol was the driving force behind forming a robust co-amorphous system with enhanced physical stability and water solubility.





Similar content being viewed by others
References
S. Lucas, J. Headache and Pain (New Jersey), 56, 436 – 446 (2016).
J. Liu, H. Grohganz, K. Löbmann, et al., Pharmaceutics, 13(3), 389 (2021).
A. Karagianni, K. Kachrimanis, I. Nikolakakis, Pharmaceutics, 10(3), 98 (2018).
M. El-Badr, G. Fetih, M. Fathy, Saudi Pharm J., 17(3), 217 – 225 (2009).
H. Takeuchi, S. Nagira, H. Yamamoto, et al., Int. J. Pharm., 293(1 – 2), 155 – 164 (2005).
T. Watanabe, I. Ohno, N. Wakiyama, et al., Int. J. Pharm., 241(1), 103 – 111 (2002).
D. Prasad, H. Chauhan, E. Atef, J. Pharm. Sci., 103(11), 3511 – 3523 (2014).
M. Alleso, N. Chieng, S. Rehder, et al., J. Control Release, 136, 45 – 53 (2009).
K. Lobmann, R. Laitinen, H. Grohganz, et al., Mol. Pharm., 8, 1919 – 1928 (2011).
A. Lodagekar, R. B. Chavan, M. K. C. Mannava, et al., Eur. J. Pharm. Sci., 139, 105048 – 105056 (2019).
K. Löbmann, C. Strachan, H. Grohganz, et al., Eur. J. Pharm. Biopharm., 81(1), 159 – 169 (2012).
S. Yamamura, H. Gotoh, Y. Sakamoto, et al., Eur. J. Pharm. Biopharm., 49(3), 259 – 265 (2000).
N. Chieng, J. Aaltonen, D. Saville, Eur. J. Pharm. Biopharm., 71, 47 – 54 (2009).
S. J. Dengale, O. P. Ranjan, S. S. Hussen, et al., Eur. J. Pharm. Sci., 62, 57 – 64 (2014).
G. G. Graham, K. F. Scott, Am. J. Th., 12(1), 46 – 55 (2005).
C. K. Ong, R. A. Seymour, P. Lirk, et al., Anesth. Analg., 110(4), 1170 – 1179 (2010).
M. Al-Remawi, A. M. A. Ali, A. Khames, et al., J. Pharm. Innov., 12(3), 206 – 215 (2017).
P. Seideman, A. Melander, Rheumatol., 27(2), 117 – 122 (1988).
M. Assali, M. Abualhasan, N. Zohud, et al., Int. J. Anal. Chem., 2020,1–9 (2020).
N. V. Dhandapani, A. A. El-gied, Int. J. Pharmacol. Pharm. Sci., 10, 1523 – 1527 (2016).
L. D. S. Alves, M. F. D. L. R Soares, C. T. de Albuquerque, et al., Polym., 104, 166 – 174 (2014).
S. Chaturvedi, M. Alim, V. K. Agrawal, Asian J. Pharm., 11, 168 – 175 (2017).
A. Modi, P. Tayade, AAPS PharmSciTech, 7(3), E87 – E92 (2006).
M. Bejaoui, H. Galai, F. Touati, et al., Dosage Forms, IntechOpen, London (2021), pp.1–14.
M. Bejaoui, H. Galai, A. B. H Amara, et al., Glass Phys. Chem., 45(6), 580 – 588 (2019).
M. Bejaoui, R. Kalfat, H. Galai, J. Pharm. Innov., 17(3), 736 – 746 (2021).
M. O’Brien, J. McCauley, E. Cohen, Analytical profiles of drug substances, Academic Press Inc., San Diego (1984), vol. 28, pp. 211 – 238.
G. Nichols, C. S. Frampton, J. Pharm. Sci., 87(6), 684 – 693 (1998).
M. K. Gupta, A. Vanwert, R. H. Bogner, J. Pharm. Sci., 92(3), 536 – 551 (2003).
H. Wen, K. R. Morris, K. Park, J. Pharm. Sci., 94, 2166 – 2174 (2005).
L. S. Taylor, G. Zografi, Pharm. Res., 14, 1691 – 1698 (1997).
X. Yuan, T. X. Xiang, B. D. Anderson, et al., Mol. Pharm., 12(12), 4518 – 4528 (2015).
H. A. Garekani, F. Sadeghi, A. Ghazi, Drug Dev. Ind. Pharm., 29, 173 – 179 (2003).
Q. Shi, F. Li, S. Yeh, et al., Int. J. Pharm., 590, 119925 (2020).
S. Desprez, M. Descamps, J. Non Cryst. Solids, 352(42 – 49), 4480 – 4485 (2006).
S. Qi, P. Avalle, R. Saklatvala, et al., Eur. J. Pharm. Biopharm., 69(1), 364 – 371 (2008).
D. Prasad, H. Chauhan, E. Atef, J. Pharm. Sci., 103(11), 3511 – 3523 (2014).
Y. Li, J. Rantanen, M. Yang, et al., Eur. J. Pharm. Sci., 129, 1 – 9 (2019).
Acknowledgements
The authors thank Pr. Abdessalem Ben Haj Amara (Faculty of Sciences of Bizerte, Tunisia) for his considerable input and helpful discussions. The authors confirmed that this research work did not receive any specific funding.
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bejaoui, M., Djemi, R., Kouass, S. et al. Co-Amorphous System (Paracetamol:Indomethacin): Investigations on Physical Stability and Intermolecular Interactions. Pharm Chem J 57, 1330–1337 (2023). https://doi.org/10.1007/s11094-024-03042-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03042-z