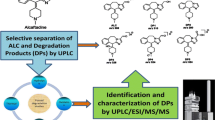
A new reverse phase ultra performance liquid chromatography method was developed and validated for the separation and analysis of impurities and zanubrutinib degradation products were characterized using liquid chromatography mass spectrometry. The chromatographic method was optimized using specimens generated by stress degradation and impurities spiked with the sample solution. On a 150 × 4.6 mm X-Bridge C18 column with a particle size of 3.5 µm connected to a PDA detector, and by using a linear mobile phase gradient prepared from trifluoroacetic acid (0.1%) in water and acetonitrile, with a flow rate of 1.0 mL/min and detection at 216 nm, good resolution of the analyte peak was obtained from peaks belonging to impurities and degradation products. Excellent accuracy, precision, and linearity results were obtained for zanubrutinbin and its impurities. When the forced test solutions were analyzed by comparison with the zanubrutinib working standard the mass balance was always close to 99.4%, indicating that the method was well stabilized and validation was performed under International Council for Harmonization requirements. Limit of detection and limit of quantification were well established, and the correlation coefficient of zanubrutinib and related compounds was 0.999.












Similar content being viewed by others
References
M. J. Rummel, N. Niederleand, G. Maschmeyer, et al., Lancet, 381(9873), 1203–1210 (2013).
H. Ye, A. Desai, S. Huang, et al., J. Exp. Clin. Cancer Res., 37(1), 150 (2018).
A. Herrmann, E. Hoster, T. Zwingers, et al., J. Clin. Oncol., 27(4), 511–518 (2009).
Z. R. Hunter, L. Xu, G. Yang, et al., Blood, 123(11), 1637–1646 (2013).
P. S. Treon, K. C. Tripsas, M. Kirsten, et al., N. Engl. J. Med., 372(15), 1430–1440 (2015).
M. Varettoni, A. Tedeschi, L. Arcaini, et al., Ann. Oncol., 23(2), 411–415 (2011).
Y. Sawalha, D. A. Bond, L. Alinari, et al., OncoTargets & Therapy, 6(13), 6573–6581(2020).
M. I. Schreuder, M. van den Brand, K. M. Hebeda, et al., J. Hematopathol., 10(3–4), 91–107 (2017).
D. M. Qato, J. Wilder, L. Schumm, et al., JAMA, 176(4), 473–482 (2016).
S. Pal Singh, F. Dammeijer, R. W. Hendriks, et al., Mol. Cancer, 17(1), 57 (2018).
C. Sacristán, M. I. Tussié-Luna, S. M. Logan, et al., J. Biol. Chem., 279(8), 7147–7158 (2004).
G. Pavlasova, Leukemia, 32(9), 2028–2031 (2018).
J. P. Deans, M. J. Polyak, Blood, 111(4), 2493–2494 (2008).
V. Dixit, Toxicol. Res., 8(2), 157–171 (2019).
L. Adamian, I. Urits, V. Orhurhu, et al., Curr. Pain Headache Rep., 24(6), 27 (2020).
S. T. Verghese, N. I. R. Riordain, R. Champaneria, et al., Int. Urogynecol. J., 2(8), 1127–1136 (2015).
A. Y. Seo, N. Kim, D. H. Oh, J. Neurogastroenterol. Motil., 19(4), 433–453(2013).
K. H. Pade, D. R. Liu, Pediatr. Emergency Med. Practice, 11(9), 1–13 (2014).
K. M. Olsen, S. Manouchehr-Pour, E. F. Donnelly, et al., J. Am. College Radiol., 17(5S), S148–S159 (2020).
M. Yuge, M. Hara, T. Hiroe, et al., Eur. Radiol., 29(2), 707–715 (2019).
S. Klahr, S. Miller, N. Engl. J. Med., 338(10), 671–675 (1998).
B. Arant, Pediatr. Nephrol., 1(3),308–13(1987).
M. Kumari Vijaya, C. H. Balasekhar Reddy, Asian J. Pharm. & Clin. Res., 13(10), 159 – 162 (2020).
T. Subrahmanyam, V. Anuradha, K. A. Prathyusha, et al., Int. J. Res. Pharm. Sci., 12(1), 808 – 814 (2021).
T. Subrahmanyam, V. Anuradha, B. S. N. Murthy, et al., J. Pharm. Res. Int., 33(31B), 203 – 211 (2021).
A. Vejendla, S. Talari, R. Moturu, et al., Fut. J. Pharm. Sci., 7, 226 (2021).
A. Vejendla, S. Talari, G. Ramu, et al., Fut. J. Pharm. Sci., 7, 234 (2021).
ICH guidelines (2003) Stability testing of new drug substances and products. Q1A (R2), current step, 4, 1 – 24.
ICH guidelines (2005) Validation of analytical procedures: text and methodology Q2 (R1). In: International conference on harmonization, Geneva, Switzerland (pp. 1 – 17).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Talari, S., Vejendla, A., Deepthi, K. et al. Development and Validation of a New Reverse Phase Ultra Performance Liquid Chromatography Method for the Estimation of Related Substances of the Anticancer Drug Zanubrutinib and Characterization of its Degradants Using Liquid Chromatography Mass Spectrometry. Pharm Chem J 57, 1118–1129 (2023). https://doi.org/10.1007/s11094-023-02992-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02992-0