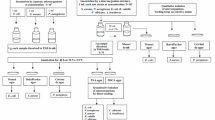
Material related to the analysis of the quality according to Microbiological Purity and Particle Size indicators of various dosage forms of ibuprofen drug and substance are presented. Possible regulatory requirements for microbiological quality are analyzed. The drug being studied is shown to affect pharmacopoeial strains of Gram-positive aerobic bacteria (B. subtilis) and yeast (C. albicans) and mold fungi (A. brasiliensis) when seeded on appropriate growth media under conditions for determining microbiological purity. The revealed antimicrobial effect was eliminated by additional dilution, which made it possible to use direct seeding for analysis. The critical quality indicator for ibuprofen suspension was Particle Size, a discrepancy of which was found in drugs for babies up to 1 year and children older than 1 year.
Similar content being viewed by others
References
T. E. Morozova, T. B. Andrushishina, and E. K. Antipova, Ter. Arkh., 85(3), 118 – 124 (2013).
https://zapis-na-priem.ru/populyarnye-stati/npvs-chto-etotakoe-v-meditsine-rasshifrovka.html
N. A. Prozorova, Candidate Dissertation in Pharmaceutical Sciences, Perm (2018).
A. S. Knyaz’kova, O. A. Semkina, and T. V. Fateeva, Fundam. Issled., 9(1), 110 – 113 (2014); https://fundamental-research.ru/ru/article/view?id=34652.
O. V. Gunar, Vedomosti NTsESMP (SCEEMP), 12(2), 120 – 123 (2022); https://doi.org/10.30895/1991-2919-2022-12-2-120-123; EDN SVKIYI.
E. S. Novik, A. V. Dorenskaya, N. A. Borisova, et al., Farmatsiya, 66(3), 8 – 11 (2017).
E. S. Novik, A. V. Dorenskaya, N. A. Borisova, et al., Farmatsiya, 66(4), 3 – 6 (2017).
E. S. Novik, A. V. Dorenskaya, N. A. Borisova, et al., Usp. Sovrem. Estestvozn., 11(2), 249 – 255 (2016).
O. V. Gunar and A. V. Dorenskaya, Farmatsiya, 71(5), 11 – 17 (2022).
SP RF, XIVth Ed.; http: // femb.ru / femb / pharmacopea.php
PEAEU, Eurasian Economic Commission, Moscow (2020).
EPh, 10th Ed., 2.6.12. Microbiological examination of nonsterile products (2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 6, pp. 53 – 58, June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gunar, O.V., Dorenskaya, A.V. & Bulgakova, G.M. Analysis of the Quality of the Drug Ibuprofen According to the Microbiological-Purity and Particle-Size Indicators. Pharm Chem J 57, 907–912 (2023). https://doi.org/10.1007/s11094-023-02966-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02966-2