Abstract
Microbial contamination of pharmaceutical preparations may cause health hazard to the patient (e.g. infection, pyrogenic or allergic reaction), altered therapeutic activity of the product, or other decrease in quality (turbidity, loss of consistency, altered pH). This chapter provides a general introduction on pharmaceutical microbiology by focusing on the essential properties of micro-organisms. First of all the basic characteristics of life and the types of biological contaminants and potentially infectious agents of pharmaceutical products will be discussed: viz. prions, viruses, mollicutes, bacteria, fungi, and endotoxins. In the next section factors affecting survival and growth of micro-organisms are discussed. In addition to well-known factors such as time, temperature, and chemical and physical characteristics of the environment, attention will be paid to biofilm formation. Primary microbiological contamination is prevented by implementing an adequate microbiological quality control and quality assurance program and by following cGMPs during production.
Microbiological quality control of pharmaceutical preparations and monitoring of production areas depend on the detection and quantification of micro-organisms. The classical, growth based, methods and some of the commercially available alternative methods are discussed.
Understanding essential microbiological concepts is necessary in designing both microbiologically stable pharmaceutical products and ensuring an effective quality control and monitoring program within the manufacturing or preparation facility.
Some of the information in this chapter has been adapted and updated from: H. van Doorne, A Basic Primer on Pharmaceutical Microbiology, edited by Richard Prince, co-published by PDA DHI Publishing Il, USA.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Characteristics of Life
Although there is no universal definition of life, scientists generally agree that living systems share all or at least the characteristics: organisation, interaction with the environment, adaptation, metabolism, growth, motility and communication.
19.1.1 Organisation
Organisms are composed of one or more cells, which are the basic units of life. Each cell must be highly organised because growth and multiplication can only occur when the individual biochemical processes are synchronised. In higher organisms, organisation within the organs, and communication with other organs are essential for the normal functioning of the body.
19.1.2 Interaction with the Environment
Cells respond to chemical and physical input from the environment. A response is often expressed by motion. Chemotaxis, the movement of a cell in response to a concentration gradient of a substance, is an example of such an interaction.
19.1.3 Adaptation
Adaptation is the accommodation of a living organism to its environment. It is fundamental to the process of evolution, by which cells change their characteristics and transmit these new properties to their offspring.
19.1.4 Metabolism
Metabolism involves the uptake of nutrients from the environment, their conversion to energy (adenosine triphosphate: ATP) and cellular components, and the deposition of waste products (e.g. carbon dioxide: CO2) or metabolites (e.g. toxins or antibiotics) into the environment. Living things require energy to maintain internal organisation (homeostasis) and to produce the other phenomena associated with life.
19.1.5 Growth
Growth is the increase in biomass. A growing individual increases up to a point in size in all of its parts. Reproduction is the result of a series of biochemical events that result in the production of a new individual (asexually, from a single parent organism, or sexually, from at least two differing parent organisms). In microbiology growth is often used as a synonym for reproduction.
Dormancy is a state of decreased metabolic activity in which there is no growth, i.e. no increase in biomass. It may be a dynamic state in which the number of newly formed cells balances the number of dying cells. The duration of this period is relatively short (hours, days). Dormancy of bacterial spores may continue for considerably longer periods of time.
19.1.6 Motility
Many cells and organisms can move under their own power. Movement to a new location may offer the cell new resources. Motility however is not a characteristic of all living organisms. Animals are typically motile, whereas plants are non-motile. In micro-organisms motility is dependent on the type of organism and sometimes even on the stage of the life cycle the cells have reached.
19.1.7 Communication
Throughout evolution humans and animals have developed a multitude of ways for communication. Micro-organisms (bacteria, yeasts and moulds) can communicate through a process called quorum sensing. Quorum sensing is the regulation of gene expression in response to fluctuations in cell-population density.
Quorum sensing exemplifies interactive social behaviour innate to the microbial world that controls features such as virulence, biofilm formation, antibiotic resistance, swarming motility, and sporulation [1, 2]. In gram-negative bacteria, small molecules (e.g. acyl homoserine lactone (ALH) [3]) serve as signals to recognise microbial cell population size. When a signal exceeds some threshold concentration the expression of specific genes is changed [4].
If a microbial cell is introduced into a pharmaceutical preparation or onto a surface it will sense whether suitable conditions (nutrients, moisture, etc.) for growth are available (interaction with the environment). If possible the cell will adapt to its new habitat, and start to metabolise the available nutrients. Eventually growth will take place. Motility of individual cells will facilitate colonisation of other sites. Production of toxins (in case of a pathogen) is a demanding biochemical process and will occur only when quorum sensing indicates that a sufficiently large population has developed.
Thus the interplay between all these characteristics determine whether a cell will be able to grow in a specific product, or on a surface.
19.2 Biological Contaminants of Pharmaceutical Preparations
Survival and growth of micro-organisms in pharmaceutical preparations is governed by extrinsic factors (particularly temperature) and intrinsic factors (product composition and physico-chemical characteristics). The combination of intrinsic and extrinsic factors will determine the types and number of micro-organisms that will develop in a product or on a surface.
19.2.1 Growth and Survival: Extrinsic Factors
19.2.1.1 Temperature
Temperature has a strong influence on whether an organism can survive or thrive. Temperature exerts its influence indirectly through water (which has to be in the liquid state), and directly through its influence on the organic molecules composing the living cells.
Mesophilic organisms are widespread in nature. They have the potential to grow in a temperature range of roughly 8–45 °C. At temperatures above 30 °C some contaminants of water and air including different types of bacteria and moulds will fail to grow or grow more slowly. The optimum growth temperature for pathogenic bacteria is around 37 °C with an upper range from 44 °C to 48 °C. Legionella pneumophila (L. pneumophila) is an exception; it will grow at temperatures up to 55 °C.
Geobacillus stearothermophilus is a thermophile and grows at temperatures between 50 °C and 65 °C. It is used as a test organism (biological indicator) to verify the efficacy of moist heat sterilisation processes.
For reasons of chemical stability some preparations must be stored frozen. Under these conditions (no liquid water) microbial growth is not possible. However, the majority of the preparations are either stored refrigerated (4–8 °C) or at room temperature (20–25 °C), which will allow growth of most mesophilic micro-organisms. According to European Good Manufacturing Practice (EU-GMP) regulations water for injection (WFI) should be produced, stored and distributed in a manner which prevents microbial growth, for example by constant circulation at a temperature above 80 °C (see also Sect. 27.5.2).
19.2.2 Growth and Survival: Intrinsic Factors
19.2.2.1 Water
The presence of water is essential to every form of life including micro-organisms. In the late 1930s, it was recognised that water activity (or aw), as opposed to water content, was the more significant factor in studying the relationship of water to microbial growth. The aw value is defined as the proportion between the water vapour pressure of the product and the vapour pressure of pure water at a common temperature.
Lowering the water content has historically been a convenient method to protect foods from microbial spoilage. Examples where the available moisture is reduced are dried fruits, syrups, and pickled meats and vegetables. Low water activity will also prevent microbial growth within pharmaceutical preparations, see also Sect. 22.3.3. Water activity values supporting microbial growth of a number of representative micro-organisms are provided in the USP [5]. That chapter also suggests a strategy for microbial limit testing based on water activity.
Pharmaceutical cleaning operations usually involve a final rinse with water of suitable pharmaceutical quality. To prevent microbial growth, it is essential to dry the object as soon as possible after rinsing.
Although water is essential for microbial growth, a low aw value does not necessarily lead to cell death. For instance, some dry raw materials, especially those of natural origin, may be heavily contaminated with both endospores and vegetative cells. Freeze drying in a suitable medium, for instance, is an excellent way to preserve pure cultures of viable micro-organisms. A water activity below 0.6 does not enable micro-organisms to grow. Solid oral dosage forms such as tablets have in general an aw value lower than 0.5 which means that these products remain stable from a microbiological point of view over long periods of time if the product is stored in a waterproof blister that remains integral.
19.2.2.2 Nutrients
Micro-organisms require for their growth suitable nutrient sources of elements (e.g. C, H, O, P, S, N), minerals (e.g. Na+, Ca2+, Mg2+) and trace elements (e.g. Cu2+, Mn2+, Co2+). Even in a relatively nutritionally poor medium such as distilled water, the number of micro-organisms may be as high as 105–106 colony forming units (CFU)/mL, indicating that nutrients are present in sufficient quantity to allow proliferation of water-borne bacteria such as the pseudomonads. The presence of readily assimilated substances such as sugars or polyalcohols in dosage forms such as creams or syrups can lead to an increased probability of microbial adulteration of those products.
19.2.2.3 pH
A pH range of 6–7 does not exert any discernible selective influence on growth of pharmaceutically relevant types of organisms. Below pH 6, growth of many bacteria will start to be inhibited. Pathogenic and toxinogenic bacteria generally will not grow at pH values below 4.5, but they may survive long times of immersion in weakly acidic solutions. Yeasts and moulds unlike bacteria, generally tolerate acidic media quite well. Optimum pH of most fungi is about pH 5. At more alkaline conditions, pseudomonads will predominate. For example, Ps. aeruginosa is known to grow at a pH range of 6–9.
19.2.2.4 Redox Potential
Microbes are classified as aerobes or anaerobes. This is now defined in terms of oxidation-reduction potential (Eh). Generally, the range at which different micro-organisms can grow are as follows: aerobes +500 to +300 mV; facultative anaerobes +300 to −100 mV; and anaerobes +100 to less than −250 mV. The redox potential of a normal aerobic nutrient medium is about + 300 mV. Thioglycolate medium, which is used for growth of anaerobic bacteria has an Eh of about −200 mV. For reasons of chemical stability, the redox potential of some pharmaceutical preparations is kept at a low level by means of reducing agents such as sulfite, tocopherol or ascorbic acid. The effect of a reduced redox potential on the microbial flora of such preparations has never been studied.
19.2.2.5 Substances with Antimicrobial Properties
The number and types of micro-organisms that may develop in various pharmaceutical dosage forms is greatly influenced by the presence of substances with antimicrobial properties. Antimicrobial active substances can be divided into three groups, as follows:
-
The first group consists of substances used for therapeutic or preventive antimicrobial purposes, for example: antibiotics, chemotherapeutics, and disinfectants.
-
The second group consists of preservatives (see Sect. 23.8), used to guarantee the microbiological quality of the product throughout its shelf life. For example: esters of hydroxybenzoic acid, quaternary ammonium substances and sorbic acid are widely used in pharmaceutical and cosmetic preparations. Other preservatives that are used include phenol, chlorhexidine, benzoic acid and benzyl alcohol.
-
The third group consists of excipients with ‘collateral’ antimicrobial activity that are principally added to dosage forms for reasons unrelated to their (sometimes weak) antimicrobial activity. For example, sodium lauryl sulfate is known to inactivate some gram-positive bacteria. Similarly, edetate has weak antimicrobial activity, and it confers synergistic antimicrobial properties when combined with quaternary ammonium substances. In addition, some active substances may show substantial antimicrobial activity.
19.3 Potential Biological Contaminants
19.3.1 Prions
19.3.1.1 Brief Description of Prions
A prion – short for proteinaceous infectious particle – is a unique type of infectious agent. Prions are composed of abnormal isoforms of a normal host-encoded membrane protein, termed prion protein (PrPc). Abnormally folded prion protein catalyses the refolding of normal prions into abnormal forms. Prions are not considered life. However, their biological origin and their potential effect on animals and human beings warrant a brief discussion.
The term ‘prion diseases’ refers to a group of neurodegenerative disorders. These include scrapie (in sheep and goat), Kuru (prion disease endemic amongst cannibalistic tribes in Papua New Guinea), bovine spongiform encephalopathy (in cattle) and Creutzfeldt-Jakob disease (CJD) (in humans) [6]. Prion diseases are characterised by long incubation periods ranging from months to years and are invariably fatal once clinical symptoms have appeared. In all prion diseases the infectious prions are generated in the brain of the afflicted animal. In the rare cases of interspecies transmission, such as from cattle to humans a ‘template assisted replication’ takes place. This means that the prions that replicate in the human brain have the amino acid sequence encoded by the DNA of the host (human being) and not the sequence of the donor animal [7].
19.3.1.2 Prions as Contaminants of Pharmaceutical Preparations
BSE was first diagnosed in the United Kingdom in 1986 and a large number of cattle and individual herds have been affected. Interspecies TSE transmission is restricted by a number of natural barriers, transmissibility being affected by the species of origin, the prion strain, dose, and route of exposure.
Transmission of scrapie to sheep and goats occurred following use of a formol-inactivated vaccine against contagious agalactia, prepared with brain and mammary gland homogenates of sheep infected with Mycoplasma agalactiae [8]. Iatrogenic transmission of human prion disease can occur through medical or surgical procedures. An example is the injection of hormones such as gonadotropins extracted from cadaver pituitaries. Current evidence indicates that, with respect to the risk of TSE infection, urinary-derived gonadotrophins appear to be safe [9]. The risks of urine-derived fertility products could now outweigh their benefits, particularly considering the availability of recombinant products [10].
Cases of CJD have also been attributed to the use of contaminated instruments in brain surgery and with the transplantation of human dura mater and cornea [11].
Suppliers of materials may minimise the risks of contamination of TSE by ensuring [12]:
-
The source animals and their geographical origin
-
Nature of animal material used in manufacture and any procedures in place to avoid cross-contamination with higher risk materials
-
Production process(es) including the quality control and quality assurance system in place to ensure product consistency and traceability
Manufacturers of pharmaceutical preparations select their raw materials so they are TSE free (see also Sect. 23.1.7). This can be ensured either by purchasing materials from non-animal origin or from non-TSE relevant animal species (e.g. porcine instead of bovine). If material from TSE-relevant animal species is purchased, it should be delivered with a certificate that confirms it free of TSE. Regular audits verifying this assumption should be performed by the manufacturer at the material’s supplier manufacturing site. Prions, unlike the normal PrPc proteins, are very resistant to inactivation. The only methods that appear to be completely effective under worst-case conditions are strong sodium hypochlorite solutions or hot solutions of sodium hydroxide [13, 14]. Some cross contamination can be avoided by the use of disposable instruments, e.g. in tonsillectomy [15].
19.3.2 Viruses
19.3.2.1 Brief Description of Viruses
A virus is a non-cellular genetic element, which is dependent on a suitable host cell for its multiplication. Their size generally ranges from 20 to 300 nm. It has been argued extensively whether viruses are living organisms. The majority of virologists consider them as non-living as they lack many of the characteristics of life, such as independent metabolism. Viruses exist in various states throughout their life cycle. In the extracellular state a virus particle is called a virion.
Virions are composed of a core of genetic material, which can either be in the form of DNA or ribonucleic acid (RNA), and a protein coat or capsid. In some viruses (enveloped viruses) the capsid is surrounded by a lipid bilayer membrane. Attached to these membranes are specific proteins, which may play a role in the attachment of the virion to the host cell, or release from the host. Thus, haemagglutinin and neuraminidase are two important enzymes present in the envelope of the influenza virus.
The virions are metabolically inactive because they are devoid of a self-generating energy system, transfer RNA, ribosomes, and so forth. Many viruses do contain enzymes that become essential in rendering these agents infectious to susceptible hosts. Viruses are obligate intracellular parasites. Replication occurs only inside the cell of a suitable host.
Replication usually leads to destruction of the host cell. Sometimes the viral DNA is incorporated into the genetic material of the host. This principle is successfully used in genetic engineering, where viruses are used as vectors to incorporate a new gene in a cell.
Viruses are causative agents of many human, animal, and plant diseases. AIDS, SARS, and avian flu are viral diseases, which are nearly daily covered by the headlines in papers and by the news items on radio and television. In 1917–1919 a ‘Spanish flu’ pandemic killed over 50 million people. The virus involved was most probably a mutation of some avian virus. The Avian flu pandemic (caused by the H5N1 variant) was, by comparison very small, as it has caused ‘only’ about 150 fatalities. The great concern for virologists and epidemiologists is the extremely high mortality rate (over 50 %) of infections with this virus. In the form of vaccines, viruses are inactivated or attenuated so as to prevent diseases in susceptible populations.
The Baltimore classification is the preferred way of classifying viruses. Viruses are grouped into families depending on their type of genome (DNA, RNA, single-stranded (ss), double-stranded (ds) etc.) and their method of replication.
A series of important medicines is derived from animal or human sources and may potentially be contaminated with undesired virus particles. Such medicines include:
-
Coagulation factors, immunoglobulins and albumin from human blood plasma
-
Vaccines and monoclonal antibodies from cell cultures
-
Proteins from cells altered by genetic engineering
-
Homoeopathic preparations of animal origin
19.3.2.2 Viruses as Contaminants of Pharmaceutical Preparations
Vaccination is one of the most important public health accomplishments. However, since vaccine preparation involves the use of materials of biological origin, such as Chinese Hamster Ovary cells, vaccines are susceptible to contamination by micro-organisms, including viruses [16–18]. Several cases of viral vaccine contamination have been reported. For example, human vaccines against poliomyelitis were found to be contaminated with SV40 virus from the use of monkey primary renal cells. Several veterinary vaccines have been contaminated by pestiviruses from foetal calf serum [19]. In 2010 the detection of fragments of a porcine circovirus was the reason for a temporary withdrawal of some commercial vaccines from the Spanish market [20].
Several methods are being used or in development to reduce infectivity of blood products, including solvent-detergent processing of plasma and nucleic acid cross-linking via photochemical reactions with methylene blue, riboflavin, psoralen and alkylating agents. Several opportunities exist to further improve blood safety through advances in infectious disease screening and pathogen inactivation methods [21, 22]. One potential way to increase the safety of therapeutic biological products is the use of a virus-retentive filter [23]. Plasma pools may be submitted to serological tests and/or genome amplification assays before they are released for further fractionation [24].
Multidose containers and the environment were found to be the source of a number of nosocomial viral infections [25–33].
Presence of viruses in pharmaceutical preparations may be verified by performing either in vivo tests by inoculating the product directly in animals (e.g. rabbits, mice) or in-vitro tests such as polymerase chain reaction (PCR) or cell culture safety tests.
19.3.3 Bacteria
19.3.3.1 Brief Description
Along with the Archea, bacteria belong to the prokaryotic organisms, i.e. cells that do not possess a real nucleus and that reproduce asexually. They are unicellular micro-organisms with a size in general ranging from 0.2 to 10 μm.
Their extraordinary diversity in terms of biochemical processes and metabolic characteristics enable bacteria to adapt themselves to a large variety of environments. Indeed, some species have the capacity to grow in anaerobic (absence of free oxygen in the air) environments by using other electron acceptors than oxygen, such as sulphates or nitrates or by fermentation. Other species may use energy and carbon sources for growth from not only organic substances but also from carbon dioxide and light energy. For this reason, bacteria have colonised most habitats on earth (soil, water, animals, plants) and even the most extreme environments such as deep-sea fumaroles or geysers.
Another fascinating (but critical in terms of product safety) characteristic of bacteria is their capacity to grow extremely fast if the environmental conditions in terms of nutrient availability, moisture and temperature become favourable.
During growth, an individual cells first increases in size and then the cell is divided in two parts (binary fission). In nutrient media, bacteria follow four growth phases (see Fig. 19.3). The first is the lag phase, during which the bacteria adapt to their new environment by repairing damaged structures and synthesising enzymes to catabolise nutrients in the medium. The second phase, the most spectacular, is the exponential phase during which nutrients in the medium are metabolised rapidly leading to a rapid doubling of the population of bacterial cells. The population of Escherichia coli cells under optimal growth conditions can multiply each 20 min. This would mean that after 8 h the population would reach one million cells and after 43 h, the volume of cells produced would be equivalent to the volume of planet earth! Once nutrients start to deplete, the exponential growth is slowed down and the amounts of cells in the overall population remains stable; this is the third phase called the stationary phase. In this phase, secondary metabolites such as antibiotics are produced in higher quantities. The last phase is when no more nutrients are available and the amount of bacterial cells starts to drop.
In the human microflora, there are at least 10 times more bacterial cells than human cells and most of them are harmless. Human bacterial infections are mainly caused by strict pathogenic species (less than 2 % of bacterial species) or by opportunistic pathogens when the immune system of the person is depleted. Bacteria may cause a large variety of infections, the most common being food poisoning, pneumonia, skin infection, urinary tract infection, throat and mouth infection, meningitis, eye infection. Depending on the infectious agent, the minimum amount of micro-organisms provoking an infectious dose varies greatly. For instance in some cases, only 10 cells of Shigella dysentriae need to be ingested to provoke dysentry but at least a 1,000 cells of Vibrio cholera to provoke cholera [34].
19.3.3.2 Mollicutes
Mollicutes, also known under the trivial name mycoplasmas, are the smallest free-living prokaryotic organisms and for years were thought to be viruses because they passed through the usual bacterial filters. They resemble protoplasts, because they lack a cell wall, but they are relatively resistant to osmotic lysis due to the presence of sterols in the cell membrane. In this respect the mycoplasmas form an exceptional group, because sterols are absent in other prokaryotic cells. Mycoplasmas are widespread in nature and many are animal, plant or human pathogens. Most mycoplasmas that infect humans are extracellular parasites. Examples of human pathogenic mycoplasmas are Mycoplasma pneumonia (infections of upper respiratory tract), and Mycoplasma genitalium (nongonococcal urethritis) [35].
Mycoplasma contamination is a major concern for vaccine and biotechnological industries since the organisms may cause disease and may interfere with cell culture [36]. Peptones, and animal sera used as components of cell culture media may be sources of this contamination [37, 38].
Mycoplasmas can be cultured in liquid or on solid media. However, in contrast with other bacteria, their growth is slow, and a microbiological assay as described in the Ph. Eur. is time-consuming (at least 28 days). Alternative, rapid methods, based on nucleic acid technologies such as PCR, have been developed [36, 39]. Under certain conditions these methods may be used as an alternative method instead of the official, growth based method [40].
19.3.3.3 Structure
Bacteria may be composed of the following structural elements (see Fig. 19.1):
-
Flagella
-
Pili and fimbriae
-
Capsule or slime layer
-
Cell wall
-
Cytoplasmic membrane
-
Cytoplasm
-
Spores
Cytoplasm, cytoplasmic membrane and cell wall are always present. The presence of the other components depends on the type of micro-organism, the culture conditions and the growth phase.
19.3.3.3.1 Flagella
Bacteria become motile by means of flagella [41]. Bacterial flagella are protein threads which originate in a defined region of the cytoplasmic membrane and protrude through the peptidoglycan layer and the outer membrane. The number of flagella per cell and their position depends on the species. Pseudomonas aeruginosa (Ps. aeruginosa) has only one (polar) flagellum at the tip of the cell, whereas Escherichia coli (E. coli) has numerous flagella spread over the entire cell surface (peritrichous). Flagella may play an important role in pathogenicity [41, 42].
19.3.3.3.2 Pili and Fimbriae
The surface of cells of some bacterial species is covered with many (10 to several thousands), thin (3–25 nm), and long (up to 12 μm) threads called pili, or fimbriae. They play a role in the initial adhesion of bacteria to host tissues and inanimate surfaces [43, 44]. Attachment to a surface is the first step in biofilm formation. Upon attachment on tissue cells they may trigger a number of biochemical signals from the host, which ultimately leads to the bacterial disease [45].
19.3.3.3.3 Capsule or Slime Layer
Capsules and slime layers – collectively called glycocalix – consist of source polysaccharide material secreted by the cell. A capsule is a rigid structure, whereas a slime layer, or loose extracellular slime, is more flexible, with diffuse boundaries. The glycocalix has several functions. It is involved in cell attachment and it may protect cells from being digested, a phenomenon known as phagocytosis. Whilst encapsulated strains of Streptococcus pneumoniae are highly pathogenic, non-encapsulated mutants are completely avirulent [46, 47]. Dextran, a slime layer product of Leuconostoc mesenteroides of relatively low molecular weight can be used as a therapeutic agent in restoring blood volume [48].
19.3.3.3.4 Cell Wall Constituents
The outer surface of the bacterial cell plays an important role in the adhesion of the cell to various surfaces. In addition to the factors that have been discussed, adhesion may also be mediated by so-called surface-associated adherence factors, usually designated as adhesins. Adhesion, which is the first step in a series of events leading to colonisation, biofilm formation and ultimately infection, is a specific process in which the adhesin “recognises” a receptor on the host surface. This specificity explains why micro-organisms such as Influenza or Mycobacterium tuberculosis can cause targeted infection of the respiratory tract but otherwise are relatively harmless when contacting other host tissues.
The cell wall gives the cell its shape and strength. The cell wall must resist the internal osmotic pressure of the cell that is estimated to be about 2 bar. The composition of cell walls of gram-positive bacteria is very different from those that stain gram-negative.
19.3.3.3.5 Gram-Positive Cell Wall
Peptidoglycan is the common cell wall component of bacteria (excluding mollicutes) and gives the wall its shape and strength. It is a polymer consisting of a backbone of alternating N-acetylglucosamine and N-acetylmuramic acid residues, cross-linked with small peptide bridges. Peptidoglycan accounts for about 80–90 % of the wall of gram-positive bacteria and for about 10 % of the gram-negative cell wall.
19.3.3.3.6 Gram-Negative Cell Wall (Outer Envelope)
The gram-negative cell wall contains only a shallow peptidoglycan layer. On the outer side of this layer is the outer membrane, a complex structure consisting of four major components: phospholipids, lipopolysaccharide, proteins (e.g., porins), and lipoprotein. Lipolysaccharide (endotoxin) is responsible for the pyrogenicity of the gram-negative bacteria.
19.3.3.3.7 Cytoplasmic Membrane
The cytoplasmic membrane, or plasma membrane is a phospholipid bilayer into which proteins/enzymes are embedded. The function of the cytoplasmic membrane is to act as a selective permeability barrier between the cytoplasm and the exterior environment. A mesosome is an organelle of bacteria that appears as an invagination of the plasma membrane and functions either in DNA replication and cell division, energy production, or excretion of exoenzymes. Flagella (if present) originate in a special structure in the cytoplasmic membrane (see the section on Flagella under Structure of the Bacterial Cell).
19.3.3.3.8 Cytoplasm
The cytoplasm is a viscous liquid, which contains all other essential elements for the living cell. The genetic material is mainly organised in the genome, a circular string of DNA. There is no discrete bacterial nucleus. The genetic code is translated into messenger RNA and then transported to the ribosomes, where the protein synthesis occurs. The building blocks of the proteins (amino acids) are transported to the ribosomes by means of transfer RNA.
Some genetic information such as antibiotic resistance may be encoded in plasmids – DNA molecules that are independent of the genome and that can replicate themselves. Some plasmids contain a set of genes (in the tra region) that enable the transfer of the plasmid by cell to cell contact (conjugation). The plasmid is replicated during this process and its genetic information (e.g. antibiotic resistance) is thus transferred to the recipient cell. There are both intra-species and inter-species plasmid transfer phenomena. The cytoplasm may also contain reserve material such as polyhydroxybutyric acid, and other substances of uncertain function (e.g. polyphosphate, volutin).
19.3.3.4 Bacterial Endospores
Some gram-positive rods such as the genera Bacillus, Geobacillus and Clostridium are capable of forming endospores that enable these genera to survive harsher conditions, such as exposure to heat, radiation, or chemicals. Bacterial spores are resistant forms of life. Some experts have suggested that they may remain viable (capable of life) for millions of years.
The bacterial spore has a complex structure, consisting of various layers, including coat and cortex, all of which play a role in long-term survival (see Fig. 19.2).
Endospore formation is a non-reproductive process: one cell produces only one spore which, after germination, produces one vegetative cell.
Bacterial sporulation occurs when growth decreases due to exhaustion of an essential nutrient. It is a complicated process requiring the participation of more than 200 enzymes. Conversion to a vegetative cell involves three steps: activation, germination and outgrowth. Activation can be accomplished by heating the spores to a non-lethal temperature. Germination can be induced by a variety of events, including exposure to nutrients (amino acids, sugars, or purine nucleosides), non-nutrient germinants (dodecylamine, lysozyme) and heating [49]. The spore loses its characteristic constituents, and heat resistance decreases dramatically. In the last stage water is taken up, and metabolism (synthesis of ATP, proteins and genetic material) resumes. Heat activation is an important factor in the occurrence of a shoulder in the survival curve of bacterial spores upon heating.
Destruction of bacterial spores is the ultimate goal of sterilisation processes. Bacterial spores are typically used in biological indicators for validation and monitoring of sterilisation processes.
19.3.4 Endotoxins/Pyrogens
Pyrogens are substances that cause a febrile reaction. Two groups of pyrogens can be distinguished: exogenous and endogenous pyrogens. The exogenous pyrogens form a heterogeneous group of substances; the most important one is lipopolysaccharide (LPS) from the cell wall of gram-negative bacteria. LPS, also known as endotoxin, has antigenic properties (O antigen) and causes fever when injected intravenously. Lipoteichoic acid, muramyldipeptide, porins, glycans and nucleic acids, are examples of non-endotoxin pyrogens originating from bacteria (gram-positive and gram-negative), yeast and moulds.
The pyrogenic activity of LPS is much higher than that of most other pyrogenic substances. This is the reason why an in-vitro limit test for LPS (the Limulus Amoebocyte Lysate, or LAL test) generally suffices for quality control purposes of parenteral medicines and raw materials, including water for injection.
The European Pharmacopoeia requires the rabbit pyrogen test for a number of vaccines, some antibiotics, and specific excipients including glucose, if intended for the preparation of large volume parenterals (see Sect. 32.8). These products may be contaminated with pyrogens other than LPS, or are known to inhibit the LAL test.
A third test, the monocyte activation test (MAT) is based on the in-vitro activation of human blood cells by pyrogens. This leads to the release of pro-inflammatory cytokines tumour necrosis factor alfa (TNF-alfa), interleukin-1 beta (IL-1beta) and interleukin-6 (IL-6) that are determined by Enzyme-Linked Immuno Sorbent Assay (ELISA). Consequently, the MAT will detect the presence of both exogenous and endogenous pyrogens in the test sample. The MAT is suitable, after a product-specific validation, as a replacement for the rabbit pyrogen test [50].
The reagent for the LAL is isolated from the blood of the horseshoe crab (Limulus polyphemus). The blood is collected from wild animals. Many animals do not survive (mortality rates of up to 30–50 % have been reported), and this living fossil is threatened with extinction. It is to be expected that in the near future the MAT test or other alternatives for the LAL test and the rabbit test will be more generally introduced. The development of such new methods will significantly reduce animal testing. The commercially most successful alternative method, which replaces the rabbit pyrogen test for bacterial impurities in medicines with a test using human cells, could save the life of 200,000 rabbits a year.
19.3.5 Biofilms
Biofilms are multicellular, microbial communities held together by a self-produced extracellular matrix that adhere to biological or non-biological surfaces [51]. A biofilm has a defined architecture, and it provides an optimal environment for the exchange of genetic material between cells, e.g. spread of antibiotic resistance. Cells within a biofilm may communicate via quorum sensing (see Sect. 19.1.7), which may in turn affect biofilm processes such as detachment of cells. The ability to form biofilms is a universal attribute of bacteria and many other micro-organisms.
Elimination of bacteria in this mode of growth is challenging due to the resistance of biofilm structures to both antimicrobials and host defences.
Biofilms have great importance for public health because of their role in certain infectious diseases and their role in a variety of device-related infections. Biofilm infections on indwelling devices or implants are difficult to eradicate because of their much better protection against macrophages and antibiotics, compared to free living cells, leading to severe clinical complications often with lethal outcome.
19.3.6 Fungi (Moulds and Yeasts)
Fungi are widespread in nature and have considerable economic and medical importance, because:
-
They may contaminate and cause spoilage and deterioration of pharmaceutical preparations. Mould and yeasts are the second cause of FDA recalls in non-sterile pharmaceuticals [52].
-
This group of organisms is used by producers of active substances, including antibiotics, such as penicillins by Penicillium species, or alkaloids, such as ergotamine by Claviceps purpurea.
-
Some fungi are pathogenic to humans. They may cause infections (e.g., Trychophyton sp. or Candida sp.), or produce toxic substances (e.g., aflatoxin by Aspergillus flavus).
Two groups of fungi are relevant in the context of pharmaceutical products or processes: the moulds and the yeasts. Their physical differentiation is not always clear, because some fungal species (e.g., Candida, Histoplasma and Cryptococcus) show dimorphism, a phenomenon in which a filamentous and a yeast-like stage both exist.
Moulds are obligate aerobic micro-organisms; they grow on the surface or in the uppermost layers of the substrate. Characteristic of moulds is the filamentous body, the mycelium. Vegetative growth of moulds occurs at the tip of the individual filaments (hyphae).
Depending on the species, hyphae may be divided into compartments by means of septa (Eumycetes). Each septum contains a pore, which allows flow of cytoplasmic constituents from one compartment to another. The lower fungi (Phycomycetes) have aseptate (coenocytic) hyphae; the mycelium is a multinucleated cell.
Asexual reproduction of moulds normally occurs by means of spore formation. From the mycelium special branches reach up into the air. At the tip of these conidiophores the spores (conidiospores) are formed on a genus specific structure. The colour of mould colonies on solid substrates (e.g., different shades of green for Penicillium species, or black for Aspergillus niger) is entirely due to the massive production of these conidiospores.
Mould spores may cause significant issues in the production of pharmaceutical preparations since they survive desiccation and may be transported via air, personnel or material flow into products.
The spores are readily dispersed into the environment and may form a new mycelium. Because of mechanical forces, such as those exerted during vortexing, hyphae may break up into smaller fragments, which may also form new mycelia. Clumps of conidiospores may also break up into smaller units. Such fragmentation caused by vigorous mixing in the course of microbiological examination of pharmaceutical samples may lead to considerable uncertainty in fungal counts.
Yeasts are typically unicellular organisms. Yeast cells are spherical or oval. Growth (asexual reproduction) takes place by a process called budding. A new cell is formed as an outgrowth of the mother cell, the daughter cell enlarges and finally the two cells separate. Pathogenic dimorphic fungi usually form yeast-like cells in the human body and a mycelium at room temperature (e.g. Histoplasma). Candida sp. is an exception because it forms hyphae in the host tissue.
Sexual reproduction is associated with many yeasts and moulds. A stage in which spores are formed is always involved in the sexual process. Depending on the type of sexual spore formation four groups of moulds can be distinguished: Ascomycetes, Basidiomycetes, Zygomycetes, and Oomycetes. The spores are called ascospores, basidiospores, zygospores, and oospores, respectively.
Fungi for which no sexual reproduction has been demonstrated are classified as fungi imperfecti (Deuteromycetes). The majority of fungi thus far classified fall into this category. Penicillium and some Aspergillus species are well-known representatives of this group.
The cell wall of fungi consists of 80–90 % polysaccharides. Chitin is a common constituent of fungal cell walls, but is replaced by other substances such as mannan, galactosan or chitosan in some species. Peptidoglycan, the common constituent of bacterial cell walls is never present. This phenomenon explains why fungi are insensitive to antibiotics that inhibit murein synthesis, such as the penicillins and the cephalosporins. Sterols are essential structural components of the fungal cytoplasmic membrane. This characteristic makes fungi sensitive to antibiotics that interact with sterols, such as nystatin and amphotericin.
19.4 Fate of Micro-organisms in Pharmaceutical Preparations
In the previous sections the characteristics of potential contaminants of pharmaceutical preparations and their requirements for survival and growth have been discussed. There appears to be a complicated interplay between the type and characteristics of the micro-organisms (e.g. spore formation), their ability to form biofilms, the composition of the environment, and external factors, particularly the temperature. The fate of a micro-organism in a pharmaceutical preparation depends on this interplay. Four different curves may be observed when numbers of colony forming units (CFU) are plotted against time (Fig. 19.3).
Microbial growth follows the well-known sigmoid growth curve, with lag phase, log phase and maximum stationary phase. Microbial destruction under influences of heat, radiation or chemicals is frequently a first order kinetic process. When microbial destruction is plotted on a semi-logarithmic scale, a straight line is observed. A ‘shoulder’ is sometimes observed at the beginning of the curve. This lower death rate is attributed to the genetic repair mechanisms of the cells, e.g. when exposed to low doses of UV radiation. Bacterial spores must be ‘activated’ before they can germinate and grow out to become prototypical vegetative cells. This phenomenon may also cause a ‘shoulder’ in survival curves. At the end of the survival curve, a ‘tail’ may be observed, indicating the presence of resistant cells or clumps of cells. True dormancy is found only in bacterial endospores. Nevertheless, even vegetative organisms can produce an effective state of dormancy because of either a relatively slow death rate or growth and kill rates that offset each other.
In pharmaceutical preparations another type of curve is sometimes observed. An initial decrease in the number of colony forming units may occur, followed by an increase. This phenomenon can be observed when analysing data from preservative efficacy testing of inadequately preserved dosage forms. It is the reason why in pharmacopoeial preservative efficacy tests the number of viable cells must be followed for a period of 28 days (see Sect. 32.8).
19.5 Biological Contamination of Pharmaceutical Preparations
19.5.1 Impact of Microbial Contamination
Microbial contamination of pharmaceutical products may result in deterioration of the product or direct hazard to the patient.
Whether a contaminated pharmaceutical product will trigger infection or disease in the patient depends on various factors such as:
-
Number of micro-organisms (CFU per g or mL)
-
Ability of the contaminant to grow and metabolise components of the product
-
Properties of the particular strain
-
Immunocompetence of the patient, due to disease (AIDS) or use of immunosuppressiva
-
Route of administration
Deterioration or spoilage of the product because of microbial growth may result in several effects including:
-
Loss of texture because of metabolism of oil/fat phase
-
Loss of organoleptic quality because of production of olfactory products
-
Loss of preservative efficacy because of metabolism of the preservative
-
Loss of package integrity because of excessive gas production
-
Loss of therapeutic activity because of metabolism of the active substance(s)
-
Release of toxigenic substances, including toxins (e.g. aflatoxin) and pyrogens
19.5.2 Origin of Microbial Contamination
The contamination can be primary or secondary. Primary contamination occurs at the premises or during preparation:
-
Personnel. Personnel account for the majority of contaminations in the clean room environments. This can be explained by the high number of micro-organisms located on or in the human body. The organisms may be introduced into the environment due to inadequate gowning or hygiene, infrequent or ineffective hand washing and disinfection procedures, unqualified behaviour (non-clean room adequate) of personnel, etc. In the aseptic production of sterile pharmaceutical preparations living micro-organisms should not enter the aseptic filling area and the product should not contain any viable micro-organism. In those situations, low-level microbial contaminations of products occur mostly at critical interventions near to the product during processing. Microbial contamination of non-sterile pharmaceutical preparations may not originate primarily from the human body, but raw materials, equipment, air and packaging material may also play an important role
-
Raw materials. Raw materials from natural origin may be highly contaminated with micro-organisms especially spore-forming bacteria and moulds and in some cases with more critical Enterobacteriaceae. Soon after a publication on salmonellosis in more than 200 persons caused by the contamination of thyroid tablets with two types of Salmonella originating from the raw material [53], proposals for the examination of non-sterile pharmaceutical preparations and acceptance criteria were published [54].
-
Water. Water may be used to clean equipment and clean rooms as well as a product component. Water contains water-borne micro-organisms that may grow under low nutrient conditions. In a recent review of FDA product recalls, almost half (48 %) of them were due to contamination by water-borne micro-organisms such as Burkholderia cepacia, Pseudomonas species, or Ralstonia pickettii [52].
-
Air. Micro-organisms may be carried over from dust or soil particles and may be transported into manufacturing areas by personnel, material or airflow. Mould spores for instance were carried over from a highly contaminated source into the production room [55].
-
Equipment. Equipment may be contaminated if inappropriate cleaning, disinfection or sterilisation procedures have been performed.
-
Primary packaging. The microbiological quality of primary packaging material is critical for sterile preparations. Vials, ampoules and stoppers shall be sterile and free of pyrogens before filling. For non-sterile preparations the microbiological quality of the packaging material is less critical. Because of the production process, bottles, tubes etc. will have only low levels of contamination, provided they have been packed, stored and handled under appropriate conditions.
Secondary contamination may occur during storage, transport, and administration of the product. The root cause for contamination during storage and transport is mainly insufficient closure integrity (see Sect. 24.3). Contamination during administration can be avoided by suitable design of the primary package (see Sect. 24.1) and appropriate instruction of the medical staff or patient (see Sect. 37.4). Tubes are less prone to contamination than jars. In hospitals eye drops should only be used for one specific patient and preferably for one specific eye.
19.5.3 Prevention of Microbial Contamination
Microbiological quality assurance and microbiological quality control should be part of the pharmaceutical quality system of any production site. Its main aim is to prevent microbial contamination in case of products required to be sterile (e.g. parenteral medicines), or to reduce the microbial counts and avoid objectionable micro-organisms in case of non-sterile products (e.g. tablets). The elements of a Pharmaceutical Quality System (PQS) that are crucial for microbiological quality regard adequate design of premises, procedures and controls. They are discussed in this section. The principles are included in current Good Manufacturing Practices (cGMP) guidelines (see Sect. 35.5.7), which are legally binding for pharmaceutical manufacturers. Microbiological quality assurance (QA) refers to the procedures in the quality system that ensures that microbiological requirements of the product are fulfilled. Microbiological quality control (QC) refers to the tests performed to verify that the product meets the required specifications.
19.5.3.1 Procedures
The following procedures and measures concerning facilities should mitigate the risk of microbiological contamination:
-
Qualified Personnel. Only trained and qualified personnel should enter areas where products are manufactured or prepared. Personnel should wear dedicated gowning which provides a physical barrier between the body and the working environment. The more critical the activity or product microbiological requirements, the stricter the gowning. Gowning may consist for instance of overalls, face masks, gloves (which are disinfected with ethanol) or even goggles in case of aseptic processing. Personnel that have apparent illnesses or infected wounds should be excluded from areas where open product is handled. Chapter Aseptic handling (Sect. 31.3.3) also discusses gowning.
-
Basic hygiene. Whereas the manufacturing of the most microbiological-critical products (e.g. parenteral, intravenous products) is strictly regulated and inspected, their preparation and application in hospitals is less subject to control. Nevertheless, measures to prevent microbial contamination or proliferation are equally, if not more important in this case. Basic hygiene rules include e.g. regular cleaning and if appropriate disinfecting the hands with alcohols, wearing sterile gloves for the preparation of parenterals, disinfecting the outer surface of critical products before opening. See Chap. 31 for detailed instructions on microbiological monitoring, disinfection procedures, operator qualification and validation of aseptic handling.
For basic hygiene at the preparation of non-sterile medicines the main points are given here.
Contact between the product and the operator is to be prevented. Thus:
-
Equipment and production processes shall be designed so that direct contact between operator and product is minimised.
-
Under no condition shall the product be touched with bare hands. If manipulation is unavoidable use utensils, such as forceps, or wear gloves. Gloves shall be changed when appropriate, particularly at every preparation and after obvious contamination such as sneezing and wiping the nose.
-
Refrain from talking above the product. Coughing and particularly sneezing are difficult to suppress. Wearing a facial mask and changing it at least every 2 h will considerably reduce the risk of contamination by this route. The operator shall inform his or her superior in case of a disease such as a cold.
-
Facial hair shall be appropriately covered; this may require the wearing of a head cover and a facial mask to cover moustaches and beards. This is also necessary from a safety point of view when operating with rotating equipment such as an ointment mill.
From a pure microbiological viewpoint wearing an overall doesn’t make sense other than the promotion of an attitude of working cleanly and neatly. Already after 1–2 h the overall bears as much contamination as the personal clothing. Directions for clothing are however also necessary to promote occupational safety and health (see Sect. 26.4.3). The overall has to remain in the preparation area (thus taken off at lunch and coffee breaks) and has to be cleaned according fixed schemes, such as daily and when visibly contaminated. The overall shall have long sleeves and cover completely personal clothing.
Washing hands must always occur:
-
At the start of preparation
-
If hands are visibly dirty
-
After using the toilets
-
After sneezing and wiping the nose
-
Between two different preparation processes, because of cross contamination
Nails have to be kept short and proper hand washing procedures include removal of watches, voluminous rings and bracelets (remaining off during the preparation process).
Washing hands technique requires preferably lukewarm water, soap from a dispenser, proper attention to thumbs, sufficient duration and proper drying with a towel because that will carry off micro-organisms too.
-
Controlled environments. Clean rooms in which pharmaceutical preparations are prepared processed, and packed are controlled for room pressure, humidity and temperature. They are segregated from other operating areas and may be entered via separate air locks for personnel and material. The incoming air of the clean rooms is filtered using HEPA (high efficiency particulate air) filters that may retain more than 99.995 % of 0.3 μm air particles (see Table 27.4). The higher the microbiological quality of the product or the critical the process step, the higher the clean room quality criteria. For instance in aseptic processing of sterile products for critical areas where the product is exposed to the surrounding environment, the air should not contain more than 3,520 particles of 0.5 μm size per cubic metre and should be devoid of viable micro-organisms (see air classifications in Tables 27.2 and 27.3). Clean room design should ensure that air, personnel and material flow is optimal to prevent microbial contamination from a less clean area to a cleaner area. Isolators have been introduced as an alternative to conventional clean rooms for aseptic production. These may be large installations, in which complex operations such as large-scale aseptic filling of syringes can be performed.
Controlled environments also regard to aseptic handling in pharmacies, where they are achieved using laminar flow cabinets, biological safety cabinets or isolators (see Sect. 28.4).
-
Cleaning and disinfection. The procedures for cleaning and disinfection (destruction of micro-organisms – but not necessarily spores – by chemical agents, see Sect. 31.4.3) of equipment parts that are in contact with the product have to be validated. In addition, for the more critical products that are required to be sterile, the equipment parts that are in contact with the product need to be sterilised. Sterilisation (destruction of micro-organisms including spores by heat) process of the manufacturing lines has also to be validated. For products, which are required to be sterile, the aseptic status of the production line is regularly evaluated by performing media fill simulations that consist of replacing the product with a microbial culture medium and evaluating if filled-media containers remain sterile.
-
Reducing bioburden. The preparation processes may reduce or even eliminate living micro-organisms. For instance on the preparation of tablets, the tableting of a granulate into a tablet may kill non-spore forming micro-organisms by the shearing forces of the interparticulate movement. Products required to be sterile are either sterile filtered (filter ≤0.2 μm pore diameter, see Sect. 30.6) or terminally sterilised directly in their container or package (e.g. steam sterilisation (Sect. 30.5.1), radiation (Sect. 30.5.3), ethylene oxide gas (30.5.4)).
-
Water distribution system. The distribution and storage systems for water that is used for cleaning, sterilisation and preparation should be devoid of biofilms. A distribution system may be controlled by continuously circulating heated water (>80 °C) in loops, avoiding one-way systems and dead ends, and application of disinfection steps such as adding ozone to the re-circulating water (see Sect. 27.5.2). The latter processes are often called sanitisation. In addition to the physico-chemical characteristics, water is monitored for microbiological counts. For preparation on a smaller scale, the storage of water should follow strict requirements as well, see further Sect. 23.3.1.
-
Standing time. Other risk mitigating actions may include defining maximum standing times for intermediate or final aqueous solutions if microbial growth is to be expected, performing internal audits to ensure that procedures are followed, and testing the product’s container closure integrity.
19.5.3.2 Tests
Microbiological testing is performed to monitor the microbiological bioburden and to ensure that the final product complies with the regulatory microbiological specifications. It comprises:
-
An environmental monitoring program in order to monitor the microbiological levels of classified rooms. Air, product-contacting surfaces, working surfaces, floors and personnel are sampled. Frequency and sampling locations are defined based on a risk assessment. Maximum microbiological count levels should be defined either based on historical data or on regulatory guidelines. If they are exceeded, this may signal a deviation from normal conditions that would require an investigation and an evaluation on the impact on the respective product produced. Trending of environmental results may also be performed in order to evaluate shifts in the overall hygienic conditions over an extended period of time to define appropriate corrective actions. See also Sect. 31.6.1.
-
Testing of primary packaging materials, raw materials (excipients, active substances, water) and products according to internal or official methods and specifications. Microbial limits of pharmaceutical preparations are given in relevant monographs of the European Pharmacopoeia. Section 19.6 provides a deeper insight on the European test methods of pharmaceutical preparations and acceptance criteria.
-
Monitoring water distribution and storage system.
-
Root cause investigation (see Sect. 35.6.15). When a test does not fulfil a microbiological acceptance criterion, this is considered as a deviation or out of specification result. This requires an investigation to determine the root cause of contamination, e.g. whether it occurred during laboratory testing, sampling or manufacturing. If a source of contamination has been found, corrective and preventive actions are put in place to eliminate and prevent re-occurrence of the contamination.
Failure to meet measures to prevent microbiological contamination of pharmaceutical preparations may have dramatic consequences. A major health issue related to microbiologically contaminated pharmaceutical preparations was the 2012 New England Compounding Center (NECC) meningitis outbreak in the USA [56]. On October 4, 2012, the CDC (US Centers for Disease Control) and the FDA (US Food and Drug Administration) issued a recall alert for pharmaceutical preparations produced by the NECC, following a multistate outbreak of fungal meningitis and other infections among patients who received contaminated preservative-free methylprednisolone acetate epidural injections. Most patients suffered infection by the fungus Exserohilum rostratum and in August 2013, CDC reported 749 cases and 63 deaths. After performing microbiological testing of the unopened incriminated product lots, CDC confirmed presence of Exserohilum rostratum, along with other fungi (e.g. Cladosporium cladosporioide, Aspergillus fumigatus) and spore-forming bacteria (e.g. Bacillus subtilis) [57]. After inspecting the NECC preparation area, the FDA mentions in its report [58] that moulds and bacteria were found in large numbers in many air and surface samples where the products were prepared.
19.5.4 Elimination and Destruction of Micro-organisms
A number of physical and chemical techniques to eliminate or to destroy micro-organisms may be employed in order to assure that the microbiological quality of the product complies with pharmacopoeial requirements, immediately after production and throughout its shelf life. Since these techniques are discussed in detail in other chapters, they are mentioned only briefly.
19.5.4.1 Physical Removal of Micro-organisms
Physical removal of micro-organisms (filtration, see Sect. 30.6) is applied for gases and liquids. High Efficiency Particulate Air (HEPA) filters are used to remove viable and non-viable particles from the air introduced in classified working areas (see Sect. 27.5.1). Hydrophobic membrane filters are used as vent filters on tanks and to filter production gases.
Membrane filtration of liquids is applied to reduce the bioburden of raw materials and of final products (see Sect. 30.6). In an aseptic process, membrane filtration is the final step before filling. During the production of a terminally sterilised product membrane filtration is applied to reduce the bioburden.
19.5.4.2 Physical Destruction of Micro-organisms
Micro-organisms may be physically destroyed by means of dry heat (see Sect. 30.5.2), moist heat (see Sect. 30.5.1) and ionising radiation (see Sect. 30.5.3).
Dry heat sterilisation is primarily applied for glassware and some heat stable raw materials. The standard temperature is between 160 °C and 180 °C. Ointments and some powders may be treated at lower temperatures because they are not stable enough. Higher temperatures (250 °C and above) are used for the depyrogenation and sterilisation of glass vials.
Moist heat sterilisation at 121 °C for 15 min is the method of choice for terminal sterilisation of finished products.
Sterilisation by means of ionising radiation of pharmaceutical preparations is not allowed in a number of countries. Many active substances and raw materials are decomposed by the doses required for sterilisation. Some polymers become brittle and glass may become discoloured. For these reasons there is only limited application for this sterilisation method for pharmaceutical preparations. Radiation sterilisation is however widely used in the medical device industry.
19.5.4.3 Chemical Destruction of Micro-organisms
Disinfection, sanitisation, decontamination, chemical sterilisation, are only a few terms for these processes. Disinfection, defined as removal, destruction or de-activation of micro-organisms on objects or surfaces, is the term of preference in this book.
Disinfection of surfaces (gloves, equipment, floors and walls) is done with a range of products, including isopropyl alcohol, ethanol, quaternary ammonium compounds, biguanides and amphoteric agents. If a sporicidal activity is required (as alcohols are not sporicidal), oxidising agents such as chlorine/hypochlorite, peracetic acid, or hydrogen peroxide may be used.
For medical devices a number of processes are available such as ethylene oxide and low-temperature hydrogen peroxide gas plasma sterilisation.
The objective of preservation is to assure the microbiological quality throughout storage and the period of use. A number of substances, including parabens, chlorhexidine, and sorbic acid are used (see Sect. 23.8).
19.6 Examination of Pharmaceutical Products
19.6.1 Sterility Test
Sterility tests appeared in the British Pharmacopoeia for the first time in 1932 and in the United States Pharmacopeia in 1936 [59]. Currently sterility testing is a legally binding release test for industrially manufactured sterile products. Only under specific conditions, including an excellent track record and a high level GMP, national authorities may grant a product and site-specific approval for parametric release (release without performing a sterility test) (see Sect. 32.8).
For many products prepared in hospital pharmacies or in institutions such as blood banks, the batch size is too small (one or only a few units) or the shelf life is too short (<14 days) to perform a complete sterility test as described in pharmacopoeias. In such instances, such as with radiopharmaceuticals (see Sect. 15.6.7), the pharmacist has to rely on the aseptic precautions during preparation (see Sect. 31.6). The use of rapid microbiological methods (RMMs) may also be an alternative to test products with such short shelf lives.
The objective of the sterility test is obviously to demonstrate that a batch is sterile, i.e. does not contain any viable micro-organism. There is an on-going debate on the rationale of the sterility test. The test is a destructive one and relatively small samples are taken from the batch. The results cannot be extrapolated to items that have not been examined. For terminally sterilised products a limit (Sterility Assurance Level, SAL of 106) has been imposed for which there is no appropriate test methodology of sufficient sensitivity. The test will only detect those micro-organisms capable of growing to detectable levels under the defined incubation conditions (media, temperatures and time). The sterility test is however the last possibility to detect any gross error in the production process. This means that applying an appropriate quality system with stable and well-controlled preparation and sterilisation processes (and not just performing a sterility test on the final product) is the key to ensure that a product that purports to be sterile remains free of microbial contamination. Regulatory initiatives strengthen the view that quality cannot be assured by final product testing, but should rather be assured by appropriate design of the manufacturing process (see Sect. 35.3). Consequently, these initiatives emphasise process understanding and monitoring over final product testing. For the way in which the process is monitored to ensure sterility during aseptic handling, see Sect. 31.6.1.
After several years of negotiation the sterility tests of the European Pharmacopoeia, The United States Pharmacopeia and the Japanese Pharmacopoeia are harmonised [59]. In the next sections the five steps of this harmonised test, as described in Ph. Eur. 2.6.1 will be discussed: sampling, sample preparation, inoculation, incubation, and interpretation. Prior to the routine application of the test, a suitability test with a range of specified test organisms shall demonstrate that growth of these organisms is not inhibited due to residual antimicrobial activity of the product.
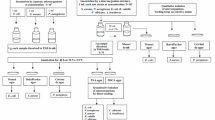
19.6.1.1 Sampling
The monograph specifies the minimum number of items that shall be examined in relationship to the batch size, and the minimum volume/amount that shall be taken from each container. The samples used should be representative for the whole batch. Furthermore immediately after an intervention in an aseptic filling operation (e.g. re-adjustment of a filling needle) the probability of contamination may be increased, and specific sampling for sterility testing may be justified. This has to be specified in relevant procedures.
19.6.1.2 Sample Preparation
Sample preparation includes all operations necessary before the actual examination can be performed, including opening of the primary packaging, withdrawal of the required amount and, if necessary, dilution or dissolution of the sample in a suitable liquid.
19.6.1.3 Inoculation
The technique of membrane filtration is used whenever the nature of the product permits. The membrane is transferred to the growth medium, or the medium is transferred onto the membrane. Alternatively, the prepared sample is inoculated directly into the appropriate media. This method is only used when the product (e.g. some vaccines) cannot be dissolved or diluted in a nontoxic diluent. The media used are fluid thioglycolate medium (FTM) for aerobic, micro-aerophilic and anaerobic bacteria, and Soybean casein digest broth (SCDB) for aerobic bacteria and fungi. FTM and SCDB are incubated at 30–35 °C and 20–25 °C respectively, both for a period of not less than 14 days. This relatively long incubation period seems to be justified, because an unacceptable proportion of contaminants would be missed by limiting incubation to 7 days [60].
19.6.1.4 Interpretation
The media are examined at intervals and at the end of the incubation period. If no growth is visually observed the sample passes the test. Normally the sample shall be rejected if growth is observed in at least one of the media. However under certain conditions the test may be invalidated and in that case be repeated.
19.6.2 Requirements for Non-sterile Products and Raw Materials
Sections 5.1.4 and 5.1.8 of the European Pharmacopoeia [61, 62] specifies microbiological quality criteria for non-sterile pharmaceutical preparations and raw materials. They are stated as Total Aerobic Microbial Count (TAMC) and Total combined Yeast and Mould Count (TYMC) (see Sect. 19.6.3) and requirements regarding specific micro-organisms. Limits depend on the route of administration, the nature of raw materials, the type of micro-organism, and on the fact whether an antimicrobial treatment can be given to the product (cf. Table 19.1 for a few examples).
The more stringent criteria for the aqueous preparations than for non-aqueous oral preparations reflect the greater potential for microbial growth of the former. E. coli must be absent from most of the preparations because this is an indicator organism for poor hygiene during production, or poor microbiological quality of the raw materials.
Products intended for inhalation must be free of Staphylococcus aureus, because it may cause pulmonary infections. This organism also indicates poor hygiene during production. Pseudomonas aeruginosa should be absent as well, because this organism may cause serious lung infections. Because of the aqueous nature of these products the presence of this organism and other bile tolerant gram-negative bacteria is a serious potential risk. Bile tolerant gram-negative bacteria form a complex group of micro-organisms comprising of the Enterobacteriaceae and many other strains (formerly Pseudomonads), including Burkholderia cepacia [63] and Ralstonia pickettii [64]. Burkholderia cepacia is a waterborne organism and causes great problems to the pharmaceutical industry and hospitals. It may be resistant to many commonly used disinfectants and preservatives, such as chlorhexidine, and it may cause serious lung infections, particularly in compromised hosts, such as cystic fibrosis patients. Salmonella must be absent from 25 g of herbal preparations. This reflects the low infectious dose of this organism and the severity of infections.
Test methods for the microbiological examination are described in Ph. Eur. Sections 2.6.12 [65] and 2.6.13 [66] respectively. Section 2.6.12 describes qualitative methods for the determination of the total aerobic microbial count and the total yeast and mould count. Section 2.6.13 describes tests for specified organisms.
19.6.3 TAMC and TYMC
The total aerobic microbial count (TAMC) is defined as the number of colonies observed on casein soya bean digest agar. The total combined yeast and mould count (TYMC) is defined as the number of colonies observed on Sabouraud-dextrose agar. The assessment consists of four steps: sampling, sample preparation/testing, incubation, and interpretation.
19.6.3.1 Sampling
In general the sample size shall be 10 g or 10 mL. An exception is made for those active substances for which the amount per dosage unit or per 1 g or 1 mL (for preparations not presented in dose units) is less than 1 mg or 1 mL. In these cases the amount to be tested shall be not less than the amount in 10 dosage units or in 10 g or 10 mL of the product.
Any sample shall be representative for the whole batch. However batch sizes may range from extremely small (e.g. for some biotechnological products) to very large (e.g. a batch of tablets). The Ph. Eur. does not describe how the samples shall be taken and this must be put down in local procedures.
19.6.3.2 Sample Preparation
If necessary the sample is dissolved and diluted in a suitable non-toxic diluent. Buffered sodium chloride-peptone solution to which an emulsifier and/or a neutraliser for antimicrobial agents may be added is widely used.
19.6.3.3 Testing/Incubation
Three methods may be used for the enumeration: membrane filtration, plate count, and most probable number (MPN) method. The advantages of the membrane filter method are its low limit of detection (LOD) of < 1 CFU/g or mL and the efficient separation of the micro-organisms from components of the product, particularly antimicrobial agents. For the pour-plate method, the sample is generally 1: 10 dissolved in the diluent, and 1 mL of the dilution is mixed with the agar. This corresponds to a LOD of 10 CFU/g or mL. The LOD is sometimes higher (e.g. 100 CFU/g or mL) if the product needs to be further diluted due to microbial inhibition, or lower in case of products with low microbial acceptance criteria. If the spread plate count technique is used the LOD is a factor of ten higher (>100 CFU/g or mL), because only 0.1 mL of the dilution can be spread over the surface of the agar plate. The precision and accuracy of the MPN method is less than that of the membrane filtration and the plate count methods. Unreliable results are particularly obtained for moulds. For these reasons the MPN method is reserved for the enumeration of TAMC in situations where no other method is available.
Casein soya bean digest agar is incubated for 3–5 days at 30–35 °C. Sabouraud dextrose agar is incubated for 5–7 days at 20–25 °C. Duplicate plates are prepared for each dilution and each medium. If the MPN method is used the tubes are incubated for 3–5 days at 30–35 °C.
19.6.3.4 Interpretation
At the end of the incubation period the colonies on the plates are enumerated. The number of CFU per gram or per mL of product is calculated for each medium from the arithmetic means of the plates. Because of the relatively poor accuracy and precision of microbiological enumerations, according to the Ph. Eur. an acceptance criterion may be interpreted as follows:
-
101 CFU: maximum acceptable count is 20
-
102 CFU: maximum acceptable count is 200; etc.
19.6.4 Specified Micro-organisms
The group of specified micro-organisms is a limited group of organisms which may be either pathogenic, or are indicator organisms for lack of hygiene during production. This group includes a number of organisms that have been discussed in a previous section, viz. Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and bile tolerant gram-negative bacteria. The group also includes: Salmonella, Clostridium, and Candida albicans. Tests for the detection of these organisms are described in the pharmacopoeias (e.g. Ph. Eur. 2.6.13 [65]).
Salmonella belongs to the family of the Enterobacteriaceae. Over 2,000 species are known. Salmonellae are differentiated by means of biochemical reactions and by serotyping. Salmonellae are pathogenic bacteria causing food poisoning. The bacterium is present in many free-living and domesticated animals. Pharmaceutical raw materials that have been contaminated include carmine, pancreatic powder and thyroid powder.
Bacteria from the genus Clostridium are anaerobic, sporeforming gram-positive rods. The spores are heat-resistant and can survive in foods that are incorrectly or minimally processed. The genus contains a number of dangerous pathogens, including Clostridium botulinum, Clostridium difficile, and Clostridium tetani.
Candida albicans is a fungus that grows both as yeast and filamentous cells and is a causal agent of oral and genital infections in humans. Systemic fungal infections including those by Candida albicans have emerged as important causes of morbidity and mortality in immunocompromised patients (e.g., patients with AIDS, cancer chemotherapy, and transplantations). Candida albicans biofilms may form on the surface of implantable medical devices. In addition, hospital-acquired infections by Candida albicans have become a cause of major health concerns.
In addition to these well-known organisms it has however been demonstrated that other organisms (e.g. Burkholderia cepacia, or Ralstonia pickettii) can cause infection when present in pharmaceutical preparations. For this reason the concept of ‘objectionable organisms’ was originally introduced in the USA. Objectionable micro-organisms are defined as contaminants that, depending on the microbiological species, would adversely affect product safety and product quality. So the assessment of microbial safety of a medicine has to include the examination of absence of objectionable micro-organisms as well. One approach to determine whether an organism is objectionable is to perform a risk analysis [55], see also Chap. 21. Such an analysis should address at least four issues:
-
Potential level of microbial contamination (total aerobic microbial count, total yeast and mould count)
-
Identity and characteristics of possible micro-organisms present (pathogenicity, ability to metabolise product components, ability to survive or even grow in the conditions of the product)
-
Product characteristics (presence of antimicrobials, water activity, route of administration, container and closure design)
-
Potential impact on patients (for instance: is the preparation meant for immunocompromised patients or for neonates)
The examination of pharmaceutical preparations for specified micro-organisms involves generally the following steps: sampling, sample preparation, resuscitation and enrichment, incubation on diagnostic or selective media, and evaluation. Sampling and sample preparation is basically the same as for TAMC and TYMC determination and will not be further discussed.
19.6.4.1 Resuscitation and Pre-enrichment
As a result of mild heat treatment, drying, or chemical antimicrobial treatment, cells may be sublethally injured. A cell is, by definition, sublethally injured if it is unable to grow on a selective medium that is typically suitable for normal healthy cells of that type. Sublethally injured cells may recover when transferred to a suitable non-selective medium and thus regain all their normal characteristics, including resistance to selective antimicrobial agents and pathogenicity. This recovery process is called resuscitation.
The examination of non-sterile pharmaceutical products for the presence of specified micro-organisms involves one or more steps with selective media. To increase the likelihood of detecting sublethally injured micro-organisms, the product is initially incubated in a non-selective (enrichment) medium. This non-selective culture thus may serve a twofold purpose: to first resuscitate sub-lethally injured cells and, secondarily, to facilitate their growth for purposes of further isolation and identification. For some organisms a second enrichment step in a selective medium is include in the method. Table 19.2 gives an overview of the purposes of the media used for each (group of) specified micro-organisms.
If there is no growth in the selective medium, the corresponding specified micro-organism is absent and the product complies with the requirement. If there is typical growth in the diagnostic medium a confirmation of the presence of the specified micro-organism should be performed using either biochemical tests or other identification methods (e.g. 16S rDNA sequencing).
19.6.5 Alternative Methods
In the previous sections only the conventional microbiological methods, developed in the late ninetieth and first half of the twentieth century, have been discussed. All these methods are growth-based methods that have their own limitations (long incubation periods, only viable micro-organisms that also grow on the media can be recovered, variability in nutrient media quality, etc.). In the past decades many new technologies have been developed with the first aim to reduce the time to obtain results of microbiological testing.
The use of such rapid microbiological methods (RMM) is beneficial in terms of reduction of throughput time for release (especially of parenterals), early identification of product contaminations, allows for causal investigations to be carried out earlier, making it easier to find and eliminate contamination causes [67]. For short shelf life products (<14 days), rapid microbiological methods are essential for assessing microbiological safety. In addition, automation by alternative methods enables to reduce hands-on time, human error and paperless data recording.
Before microbiological testing can be performed with an alternative method, the user must demonstrate that the alternative method is suitable for its intended purpose. This validation is based on demonstrating at least equivalent performance of the RMM compared to the traditional method and is performed according to Ph. Eur. 5.1.6., USP <1,223 > or PDA TR-33 [68–70].
It is beyond the scope of this chapter to discuss all available technologies, just one example of each of three detection principles will be mentioned here, viz., a growth based method, a non-growth based method and a nucleic acid determination method. For an encyclopaedic overview the reader is referred to the literature [71].
19.6.5.1 Growth Based Method: ATP Bioluminescence
ATP is a metabolite present in all organisms (excluding viruses). The amount of ATP per cell is species-dependent and also is dependent on the metabolic state of the cell. ATP can be measured semi-quantitatively with the luciferin/luciferase system. This system (naturally occurring in the firefly) emits light in the presence of ATP. The sample is mixed with a special reagent, which causes lysis of the cells, liberating the ATP, followed by addition of the luciferin and luciferase reagents. The amount of emitted light is measured by means of a photomultiplier system and used as an indicator of the number of cells present. This principle can be applied for numerous purposes, such as the detection of micro-organisms in products or monitoring of cleaning and disinfection procedures. Its use has been suggested for microbiological examination of sterile and non-sterile pharmaceutical preparations [72, 73].
19.6.5.2 Non-growth Based Method: Solid State Laser Scanning Cytometry
A sample is filtered over a membrane filter and treated with a fluorogenic reagent. Living cells actively take up the reagent and convert it to a fluorescent substance. The filter is scanned with a laser beam and the fluorescence is measured. The system records the position of the fluorescent item, so that it can be verified microscopically that the signal was not due to an artefact. The method is able to detect a single cell within about 2 h post sample processing and testing. It is used to monitor large water systems in pharmaceutical plants [74].
19.6.5.3 Nucleic Acid Based Identification: Ribotyping
Ribotyping measures the pattern that is generated when DNA from an organism is treated with a restriction enzyme. DNA is isolated from a pure culture. The genes encoding for 16S rRNA are amplified with PCR, treated with one or more restriction enzymes, separated by means of electrophoresis and finally probed. The obtained genetic fingerprint is a stable marker that provides definitive species discrimination or even characterisation below species level.
References
Bandara HM, Lam OL, Jin LJ, Samaranayake L (2012) Microbial chemical signaling: a current perspective. Crit Rev Microbiol 38:217–249
Mangwani N, Dash HR, Chauhan A, Das S (2012) Bacterial quorum sensing: functional features and potential applications in biotechnology. J Mol Microbiol Biotechnol 22:215–227
Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR (2011) Quorum sensing in gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev 111:28–67
Thoendel M, Horswill AR (2010) Biosynthesis of peptide signals in gram-positive bacteria. Adv Appl Microbiol 71:91–112
The United States Pharmacopeial USP 37/The National Formulary NF 32 (2014) <1112>, application of water activity determination to non-sterile pharmaceutical products. The United States Pharmacopeial Convention, Rockville, pp 925–926
Haltia M (2000) Human prion diseases. Ann Med 32:493–500
Baron HS, Groth D, DeArmond SJ, Prusiner SB (2001) Prions. In: Block SS (ed) Disinfection, sterilization and preservation. Lippincott, Philadelphia
Bertolini S, Maurella C, Bona C, Ingravalle F, Desiato R, Baioni E, Chiavacci L, Caramelli M, Ru G (2012) A relevant long-term impact of the circulation of a potentially contaminated vaccine on the distribution of scrapie in Italy. Results from a retrospective cohort study. Vet Res 43:63–72
Balen AH, Lumholtz IB (2005) Consensus statement on the bio-safety of urinary-derived gonadotrophins with respect to Creuzfeldt-Jakob disease. Hum Reprod 20:2994–2999
Van Dorsselaer A, Carapito C, Delalande F, Schaeffer-Reiss C, Thierse D, Diemer H, McNair DS, Krewski D, Cashman NR (2011) Detection of prion protein in urine-derived injectable fertility products by a targeted proteomic approach. PLoS One 6:e17815
Alter M (2000) How is Creutzfeldt-Jakob disease acquired? Neuroepidemiology 19:55–61
Ph. Eur (2014) Minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 2.1.8
Taylor DM (2000) Inactivation of transmissible degenerative encephalopathy agents: a review. Vet J 159:10–17
Appel T, Lucassen R, Groschup MH, Joncic M, Beekes M, Riesner D (2000) Acid inactivation of prions: efficient at elevated temperature or high acid concentration. J Gen Virol 87:1385–1394
Department of Health (2001) Re-introduction of re-usable instruments for tonsil surgery. Department of Health, London
Kumar D, Beach NM, Meng XJ, Hegde NR (2012) Use of PCR-based assays for the detection of the adventitious agent porcine circovirus type 1 (PCV1) in vaccines, and for confirming the identity of cell substrates and viruses used in vaccine production. J Virol Methods 179:201–211
Chen D, Nims R, Dusing S, Miller P, Luo W, Quertinmont M, Parekh B, Poorbaugh J, Boose JA, Atkinson EM (2008) Root cause investigation of a viral contamination incident occurred during master cell bank (MCB) testing and characterization–a case study. Biologicals 36:393–402
Kekarainen T, Martinez-Guino L, Segales J (2009) Swine torque teno virus detection in pig commercial vaccines, enzymes for laboratory use and human drugs containing components of porcine origin. J Gen Virol 90:648–653
Pastoret PP (2010) Human and animal vaccine contaminations. Biologicals 38:332–334
Bouzon AM, Diez DJ, Martinon-Torres F (2011) Circovirus and impact of temporary withdrawal of rotavirus vaccines in Spain. Hum Vaccin 7:798–799
Lindholm PF, Annen K, Ramsey G (2011) Approaches to minimize infection risk in blood banking and transfusion practice. Infect Disord Drug Targets 11:45–56
Nubling CM, Unkelbach U, Chudy M, Seitz R (2008) Effect of viral nucleic acid testing on contamination frequency of manufacturing plasma pools. Transfusion 48:822–826
Kim IS, Choi YW, Kang Y, Sung HM, Sohn KW, Kim YS (2008) Improvement of virus safety of an antihemophilc factor IX by virus filtration process. J Microbiol Biotechnol 18:1317–1325
Groner A (2008) Pathogen safety of plasma-derived products – Haemate P/Humate-P. Haemophilia 14(Suppl 5):54–71
Hamada N, Gotoh K, Hara k, Iwahashi J, Imamura Y, Nakamura S, Taguchi C, Sugita M, Yamakawa R, Etoh Y, Sera N, Ishibashi T, Chijiwa K, Watanabe H (2008) Nosocomial outbreak of epidemic keratoconjunctivitis accompanying environmental contamination with adenoviruses. J Hosp Infect 68:262–268
Kowalski RP, Romanowski EG, Waikhom B, Gordon YJ (1998) The survival of adenovirus in multidose bottles of topical fluorescein. Am J Ophthalmol 126:835–836
Patel PR, Larson AK, Castel AD, Ganova-Raeva LM, Myers RA, Roup BJ, Farrell KP, Edwards L, Nainan O, Krick JP, Blythe D, Fiore AE, Roche JC (2006) Hepatitis C virus infections from a contaminated radiopharmaceutical used in myocardial perfusion studies. J Am Med Assoc 296:2005–2011
Branch-Elliman W, Weiss D, Balter S, Bornschlegel K, Phillips M (2013) Hepatitis C transmission due to contamination of multidose medication vials: summary of an outbreak and a call to action. Am J Infect Control 41:92–94
Moore ZS, Schaefer MK, Hoffmann KK, Thompson SC, Xia GL, Lin Y, Khudyakov Y, Maillard JM, Engel JP, Perz JF, Patel PR, Thompson ND (2011) Transmission of hepatitis C virus during myocardial perfusion imaging in an outpatient clinic. Am J Cardiol 108:126–32
Germain JM, Carbonne A, Thiers V, Gros H, Chastan S, Bouvet E, Astagneau P (2005) Patient-to-patient transmission of hepatitis C virus through the use of multidose vials during general anesthesia. Infect Control Hosp Epidemiol 26:789–792
Krause G, Trepka MJ, Whisenhunt RS, Katz D, Nainan O, Wiersma ST, Hopkins RS (2003) Nosocomial transmission of hepatitis C virus associated with the use of multidose saline vials. Infect Control Hosp Epidemiol 24:122–127
Romanowski EG, Barnhorst DA, Kowalski RP, Gordon YJ (1999) The survival of herpes simplex virus in multidose office ophthalmic solutions. Am J Ophthalmol 128:239–240
Kidd-Ljunggren K, Broman E, Ekvall H, Gustavsson O (1999) Nosocomial transmission of hepatitis B virus infection through multiple-dose vials. J Hosp Infect 43:57–62
Forsythe SJ (2000) The microbiology of safe food. Blackwell Science, Oxford
Razinand S, Hayflick L (2010) Highlights of mycoplasma research–an historical perspective. Biologicals 38:183–190
Armstrong SE, Mariano JA, Lundin DJ (2010) The scope of mycoplasma contamination within the biopharmaceutical industry. Biologicals 38:211–213
Windsor HM, Windsor GD, Noordergraaf JH (2010) The growth and long term survival of Acholeplasma laidlawii in media products used in biopharmaceutical manufacturing. Biologicals 38:204–210
Cheng HS, Shen CW, Wang SR (2007) Effect of storage conditions on detection of mycoplasma in biopharmaceutical products. In Vitro Cell Dev Biol Anim 43:113–119
Volokhov DV, Graham LJ, Brorson KA, Chizhikov VE (2011) Mycoplasma testing of cell substrates and biologics: review of alternative non-microbiological techniques. Mol Cell Probes 25:69–77
Ph. Eur (2014) Mycoplasmas. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 2.6.7
Moens S, Vanderleyden J (1996) Functions of bacterial flagella. Crit Rev Microbiol 22:67–100
Penn CW, Luke CJ (1992) Bacterial flagellar diversity and significance in pathogenesis. FEMS Microbiol Lett 79:331–336
Pizarro-Cerda J, Cossart P (2006) Bacterial adhesion and entry into host cells. Cell 124:715–727
Telford JL, Barocchi MA, Margarit I, Rappuoli R, Grandi G (2006) Pili in gram-positive pathogens. Nat Rev Microbiol 4:509–519
Sauer FG, Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ (2000) Bacterial pili: molecular mechanisms of pathogenesis. Curr Opin Microbiol 3:65–72
Llull D, Lopez R, Garcia E (2001) Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr Mol Med 1:475–491
Makela PH (2003) Conjugate vaccines–a breakthrough in vaccine development. Southeast Asian J Trop Med Public Health 34:249–253
Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ (2005) Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol 80:845–860
Setlow P (2003) Spore germination. Curr Opin Microbiol 6:550
Ph. Eur (2014) Monocyte-activation test. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 2.6.30
Lopez D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2:a000398
Jimenez L (2007) Microbial diversity in pharmaceutical product recalls and environments. PDA J Pharm Sci Technol 61:383–399
Kallings LO, Ringertz O, Silverstolpe L (1966) Microbiological contamination of medical preparations. Acta Pharm Suec 3:219–228
Buhlmann X, Gay M, Gubler HU, Hess H, Kabay A, Knusel F, Sackman W, Schiller I, Urban S (1972) Microbiological quality of pharmaceutical preparations. Guidelines and methods. Am J Pharm Sci Support Public Health 144:165–186
Roesti D (2012) Objectionable micro-organisms in non-sterile pharmaceutical drug products: risk assessment and origins of contamination. In: Moldenhauer J (ed) Environmental monitoring, vol 6. PDA/DHI, Bethesda
Anonymous. Multistate fungal meningitis outbreak investigation. http://www.cdc.gov/hai/outbreaks/meningitis.html. Accessed 26 Sept 2013
Smith RM, Schaefer MK, Kainer MA, Wise M, Finks J et al. (2013) Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med 369:1598–1609
FDA report on New England Compounding Pharmacy. www.fda.gov search on UCM325980
Bowman FW (1969) The sterility testing of pharmaceuticals. J Pharm Sci 58:1301–1307
Bathgate H, Lazzari D, Cameron H, McKay D (1993) The incubation period in sterility testing. J Parenter Sci Technol 47:254–257
Ph. Eur (2014) Microbiological quality of pharmaceutical preparations. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 5.1.4
Ph. Eur (2014) Microbiological quality of herbal medicinal products for oral use. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 5.1.4
Torbeck L, Raccasi D, Guilfoyle DE, Friedman RL, Hussong D (2011) Burkholderia cepacia: this decision is overdue. PDA J Pharm Sci Technol 65:535–543
Labarca JA, Trick WE, Peterson CL, Carson LA, Holt SC, Arduino MJ, Meylan M, Mascola L, Jarvis WR (1999) A multistate nosocomial outbreak of Ralstonia pickettii colonization associated with an intrinsically contaminated respiratory care solution. Clin Infect Dis 29:1281–1286
Ph. Eur (2014) Microbiological examination of non-sterile products: microbial enumeration tests. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 2.6.12
Ph. Eur (2014) Microbiological examination of non-sterile products: test for specified micro-organisms. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 2.6.13
Gordon OG, Gray JC, Anders HJ, Staerk A, Neuhaus G, Schaefli O (2011) Overview of rapid microbiological methods evaluated, validated and implemented for microbiological quality control. Eur Pharm Rev 2:9–13
Ph. Eur (2014) Alternative methods for control of microbiological quality. European Pharmacopoeia, 8th edn. Council of Europe, Strasbourg, 5.1.6
The United States Pharmacopeial USP 37/The National Formulary NF 32 (2014) <1223>, validation of alternative microbiological methods. The United States Pharmacopeial Convention, Rockville, pp 1152–1155
Anonymous (2013) PDA Technical Report #33, Evaluation, Validation and Implementation of New Microbiological Testing Methods PDA. Bethesda
Miller MJ (ed) (2005) Encyclopedia of rapid microbiological methods. PDA DHI, Bethesda
Gray JC, Staerk A, Berchtold M, Hecker W, Neuhaus G, Wirth A (2010) Growth promoting properties of different solid nutrient media evaluated with stressed and unstressed micro-organisms: prestudy for the validation of a rapid sterility test. PDA J Pharm Sci Technol 64:249–263
Gray JC, Morandell G, Gapp G, Le Goff N, Neuhaus G, Staerk A (2011) Identification of micro-organisms after milliflex rapid detection–a possibility to identify nonsterile findings in the milliflex rapid sterility test. PDA J Pharm Sci Technol 65:42–54
Wallner G, Tillmann D, Haberer K (1999) Evaluation of the ChemScan system for rapid microbiological analysis of pharmaceutical water. PDA J Pharm Sci Technol 53:70–74
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 KNMP and Springer International Publishing Switzerland
About this chapter
Cite this chapter
van Doorne, H., Roesti, D., Staerk, A. (2015). Microbiology. In: Bouwman-Boer, Y., Fenton-May, V., Le Brun, P. (eds) Practical Pharmaceutics. Springer, Cham. https://doi.org/10.1007/978-3-319-15814-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-15814-3_19
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15813-6
Online ISBN: 978-3-319-15814-3
eBook Packages: MedicineMedicine (R0)