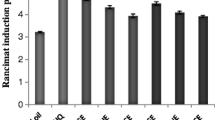
Bioactive compounds of cornelian cherry (Cornus mas L.) fruits were extracted by green extraction methods including ultrasound-assisted (with three solvents: water, water/ethanol 50:50 v/v, and ethanol), supercritical fluid, and subcritical water extraction. Evaluation of the flavonoid and tocopherol compounds, β-carotene/linoleic acid test, and oil oxidative stability index assay were used to quantify the antioxidant power of cornelian cherry extracts. In β-carotene/linoleic acid test, a strong antioxidant activity of 85.84% was observed in the water/ethanol (50:50, v/v) ultrasound assisted extract, which also had the highest extraction yield and total flavonoid and tocopherol contents achieved by this method. In oil oxidative stability index assay, cornelian cherry extracts had higher resistance to oxidation than the tert-butylhydroquinone (TBHQ) synthetic antioxidant. Among the extracts, the longest induction time of 4.50 h belonged to water/ethanol (50:50, v/v) ultrasound-assisted extract. High and significant correlation was found between the extracted components and antioxidant capacity. This research demonstrated that the cornelian cherry fruit can be used as a potential source of natural antioxidant in food industry.


Similar content being viewed by others
References
C. A. Rashid, M. Zahid Qureshi, S. A. Raza, et al., Univ. Bucuresti. Chimie., 19(1), 23 – 30 (2010).
R. Esmaeilzadeh Kenari, F. Mohsenzadeh, and Z. Raftani Amiri, Food Sci. Nutr., 2(4), 426 – 435 (2014).
R. Sushma, H. Dharini, T. Sadiya, et al., Res. J. Pharm., Biol. Chem. Sci., 5(4), 624 – 632 (2014).
Z. Huyut, F. Beydemir, and E. Gulcin, Biochem. Res. Int., 1 – 10 (2017).
N. Hassim, M. Markom, N. Anuar, et al., Int. J. Chem. Eng., 1 – 10 (2015).
O. Rop, J. Mlcek, D. Kramarova, and T. Jurikova, Afr. J. Biotechnol., 9(8), 1205 – 1210 (2010).
M. Narimani-Rad, M. Zendehdel, M. Mesgari Abbasi, et al., Bull. Environ., Pharmacol. Life Sci., 2(9), 48 – 50 (2013).
M. S. Stankovic, M. Zia-Ul-Haq, B. M. Bojovic, and M. D. Topuzovic, Bulg. J. Agric. Sci., 20(2), 358 – 363 (2014).
H. Hassanpour, Y. Hamidoghli, J. Hajilo, and M. Adlipour, Sci. Hortic., 129(3), 459 – 463 (2011).
R. Vidrih, Ž., Čejić, and J. Hribar, Croat. J. Food Sci. Technol., 4(1), 64 – 70 (2012).
T. Dhanani, S. Shah, N. A. Gajbhiye, and S. Kumar, Arab. J. Chem., 10(1), 1193 – 1199 (2013).
J. Wiboonsirikul and S. Adachi, Food Sci. Technol. Res., 14(4), 319 – 328 (2008).
A. Roidaki, P. G. Zoumpoulakis, and C. Proestos, Austin J. Nutri. Food Sci., 3(1), 1 – 8 (2015).
T. W. Charpe and V. K. Rathod, Iran. J. Chem. Eng., 11(4), 21 – 30 (2014).
G. N. Sapkale, S. M. Patil, U. S. Surwase, and P. K. Bhatbhage, Int. J. Chem. Sci., 8(2), 729 – 743 (2010).
P. Garcia-Salas, A. Morales-Soto, A. Segura-Carretero, and A. Fernandez-Gutierrez, Molecules, 15(12), 8813 – 8826 (2010).
H. Liu, Z. Jiao, J. Liu, et al., Food Anal. Methods, 6(3), 781 – 788 (2013).
W. Poontawee, S. Natakankitkul, and O. Wongmekiat, J. Anal. Methods Chem.,1 – 7 (2015).
M. Hassas-Roudsari, P. R. Chang, R. B. Pegg, and R. T. Tyler, Food Chem., 114(2), 717 – 726 (2009).
S. Tunchaiyaphum, M. N. Eshtiaghi, and N. Yoswathana, Int. J. Chem. Eng. Appl., 4(4), 194 – 198 (2013).
A. Shotipruk, J. Kiatsongserm, P. Pavasant, et al., Biotechnol. Prog., 20(6), 1872 – 1875 (2004).
A. H. Goli, M. Barzegar, and M. A. Sahari, Food Chem., 92(3), 521 – 525 (2005).
M. Delfanian, R. Esmaeilzadeh Kenari, and M. A. Sahari, Food Sci. Nutr., 3(3), 179 – 187 (2015).
C. C. Chang, M. H. Yang, H. M. Wen, and J. C. Chern, J. Food Drug Anal., 10(3), 178 – 182 (2002).
M. L. Wong, R. E. Timms, and E. M. Goh, J. Am. Oil Chem. Soc., 65(2), 258 – 261 (1988).
A. Dapkevicius, R. Venskutonis, T. A. V. Beek, and J. P. H. Linssen, J. Sci. Food Agric., 77(1), 140 – 146 (1998).
J. M. Duarte-Almeida, R. J. D. Santos, M. I. Genovese, and F. M. Lajolo, Ciênc. Tecnol. Aliment., 26(2), 446 – 452 (2006).
R. Farhoosh, and M. H. Tavassoli-Kafrani, Food Chem., 125(1), 209 – 213 (2011).
E. A. Hayouni, M. Abedrabba, M. Bouix, and M. Hamdi, Food Chem., 105, 1126 – 1134 (2007).
S. Felhi, A. Daoud, H. Hajlaoul, et al., Food Sci. Technol., 37(3), 483 – 492 (2017).
N. V. Yanishlieva–Maslarova, in: Antioxidants in food: practical applications, J. Pokorny, N. Yanishlieva, and M. Gordon, (Eds.), Cambridge: CRC Press–Woodhead Publishing (2001), pp. 22 – 70.
M. Bimakr, R. A. Rahman, F. S. Taip, et al., Food Bioprod. Process., 89, 67 – 72 (2011).
M. A. Rostagno, M. Palma, and C. G. Barroso, J. Chromatogr. A., 1012(2), 119 – 128 (2003).
S. H. Rouhani, N. Alizadeh, S. H. Salimi, and T. Haji-Ghasemi, Prog. Color, Color. Coat., 2, 103 – 113 (2009).
R. F. Susanti, K. Kurnia, A. Vania, and I. J. Reynaldo, Mod. Appl. Sci., 9(7), 190 – 198 (2015).
A. Sharifi, S. A. Mortazavi, A. Maskooki, et al., Int. J. Agric. Crop Sci., 6(2), 89 – 96 (2013).
T. Ogunmoyole, O. O. Olalekan, O. Fatai, et al., J. Pharmacogn. Phytother., 4(5), 66 – 74 (2012).
C. Sun, Z. Wu, Z. Wang, and H. Zhang, J. Evidence-Based Complementary. Altern. Med., 1 – 9 (2015).
E. E. Ezeokeke, A. C. Ene, and C. U. Igwe, Biochem. Anal. Biochem., 4(4), 1 – 4 (2015).
A. Ghasemzadeh, H. Z. E. Jaafar, A. S. Juraimi, and A. Tayebi-Meigooni, Molecules, 20(6), 10822 – 10838 (2015).
M. Herrero, M. Castro-Puyana, J. A. Mendiola, and E. Ibanez, Trends Anal. Chem., 43, 67 – 83 (2012).
N. Yoswathana, M. N. Eshiaghi, and K. Jaturapornpanich, Int. J. Chem. Mol. Nucl. Mater. Metal. Eng., 6(5), 453 – 459 (2012).
K. Pycia, I. Kapusta, G. Jaworska, and A. Jankowska, Eur. Food Res. Technol., 1 – 10 (2018).
A. H. Goli, F. Mokhtari, and M. Rahimmalek, J. Agric. Sci., 4(10), 175 – 181 (2012).
A. Luis, N. Gil, M. E. Amaral, et al., BioResources, 7(2), 2105 – 2120 (2012).
N. Alam, K. N. Yoon, J. S. Lee, et al., Saudi J. Biol. Sci., 19(1), 111 – 118 (2012).
H. M. Hadzri, M. A. C. Yunus, S. Zhari, and F. Rithwan, J. Teknol., 68(5), 47 – 52 (2014).
N. Wu, K. Fu, Y. Fu, et al., Molecules, 14(3), 1032 – 1043 (2009).
M. L. Colombo, Molecules, 15(4), 2103 – 2113 (2010).
T. Kulisic, A. Radonic, V. Katalinic, and M. Milos, Food Chem., 85(4), 633 – 640 (2004).
M. Abedi Gonabad, M. Shahidi Noghabi, and R. Niazmand, J. Appl. Environ. Biol. Sci., 4, 28 – 32 (2015).
N. Ozturk, Pak. J. Pharm. Sci., 28(2), 465 – 472 (2015).
N. Pawar, K. Gandhi, A. Purohit, et al., J. Food Sci. Technol., 51(10), 2727 – 2733 (2014).
R. Upadhyay and H. N. Mishra, Ind. Crops Prod., 61, 453 – 459 (2014).
S. Yassari and E. Yasari, Int. J. Agric. Crop Sci., 5(4), 410 – 420 (2013).
S. C. Ren, Z. L. Liu, and P. Wang, J. Med. Plants Res., 6(2), 301 – 308 (2012).
M. A. Naeem, H. A. Zahran, and M. M. M. Hassanein, OCL: Oilseeds Fats, Crops Lipids, 26(33), 1 – 9 (2019).
Y. Y. Lee, H. M. Park, T. Y. Hwang, et al., J. Sci. Food Agric., 1 – 9 (2014).
J. Lalièić-Petronijević, D. Komes, S. Gorjanoviæ, et al., Food Technol. Biotechnol., 54(1), 13 – 20 (2016).
S. Noureen, S. Noreen, S. A. Ghumman, et al., Not. Bot. Horti Agrobot. Cluj-Napoca, 46(2), 653 – 662 (2018).
Acknowledgments
The authors are grateful to the Sari Agricultural Sciences and Natural Resources University for their support in this research work.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gillani, F., Raftani Amiri, Z. & Esmaeilzadeh Kenari, R. Assay of Antioxidant Activity and Bioactive Compounds of Cornelian Cherry (Cornus mas L.) Fruit Extracts Obtained by Green Extraction Methods: Ultrasound-Assisted, Supercritical Fluid, and Subcritical Water Extraction. Pharm Chem J 56, 692–699 (2022). https://doi.org/10.1007/s11094-022-02696-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02696-x